

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Keq and ΔG0 Are Measures of a Reaction’s Tendency to Proceed Spontaneously
المؤلف:
David L. Nelson, Michael M. Cox
المصدر:
Lehninger Principles of Biochemistry 6th ed 2012
الجزء والصفحة:
6th ed -p26
7-8-2016
2125
Keq and ΔG0 Are Measures of a Reaction’s Tendency to Proceed Spontaneously
The tendency of a chemical reaction to go to completion can be expressed as an equilibrium constant.
aA + bB → cC + dD
For the reaction the equilibrium constant, Keq, is given by
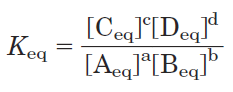
where [Aeq] is the concentration of A, [Beq] the concentration of B, and so on, when the system has reached equilibrium. A large value of Keq means the reaction tends to proceed until the reactants have been almost completely converted into the products.
Gibbs showed that ΔG for any chemical reaction is a function of the standard free-energy change, ΔG0— a constant that is characteristic of each specific reaction—and a term that expresses the initial concentrations of reactants and products:
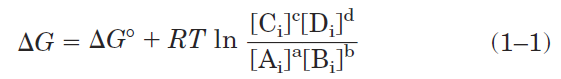
where [Ai] is the initial concentration of A, and so forth; R is the gas constant; and T is the absolute temperature. When a reaction has reached equilibrium, no driving force remains and it can do no work: ΔG = 0. For this special case, [Ai] = [Aeq], and so on, for all reactants and products, and
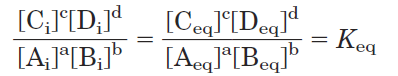
Substituting 0 for ΔG and Keq for [Ci]c[Di]d/[Ai]a[Bi]b in Equation 1–1, we obtain the relationship
ΔG0 = -RT ln Keq
from which we see that ΔG0 is simply a second way (besides Keq) of expressing the driving force on a reaction. Because Keq is experimentally measurable, we have a way of determining ΔG0, the thermodynamic constant characteristic of each reaction. The units of ΔG0 and ΔG are joules per mole (or calories per mole). When Keq >> 1, ΔG0 is large and negative; when Keq << 1, ΔG0 is large and positive. From a table of experimentally determined values of either Keq or ΔG0, we can see at a glance which reactions tend to go to completion and which do not. One caution about the interpretation of ΔG0: thermodynamic constants such as this show where the final equilibrium for a reaction lies but tell us nothing about how fast that equilibrium will be achieved. .
 الاكثر قراءة في مواضيع عامة في الكيمياء الحياتية
الاكثر قراءة في مواضيع عامة في الكيمياء الحياتية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















