


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 1-12-2019
Date: 28-7-2019
Date: 30-10-2019
|
Meso Compounds
Let’s look at another example of a compound with more than one chirality center: the tartaric acid used by Pasteur. The four stereoisomers can be drawn
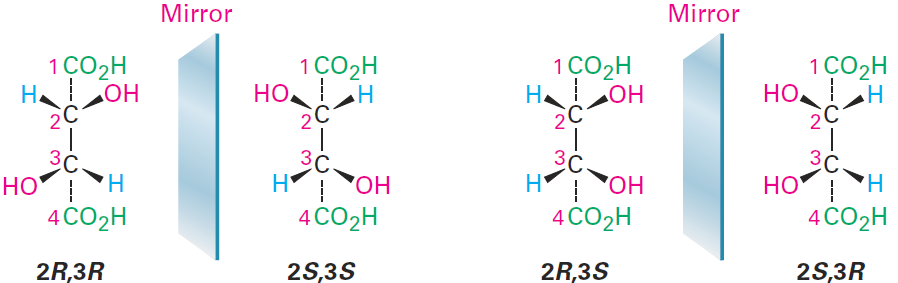
The 2R,3R and 2S,3S structures are nonsuperimposable mirror images and therefore represent a pair of enantiomers. A close look at the 2R,3S and 2S,3R structures, however, shows that they are superimposable, and thus identical, as can be seen by rotating one structure 180°.
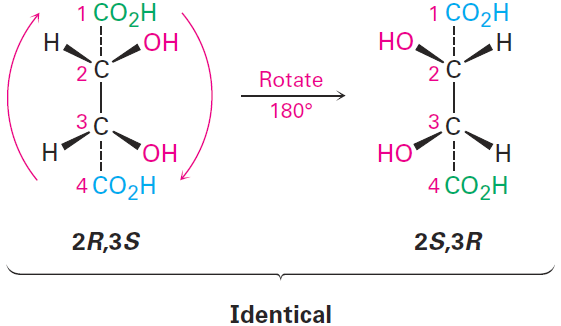
The 2R,3S and 2S,3R structures are identical because the molecule has a plane of symmetry and is therefore achiral. The symmetry plane cuts through the C2–C3 bond, making one half of the molecule a mirror image of the other half (Figure 1-1). Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. Compounds that are achiral, yet contain chirality centers, are called meso compounds (me-zo). Thus, tartaric acid exists in three stereoisomeric forms: two enantiomers and one meso form.
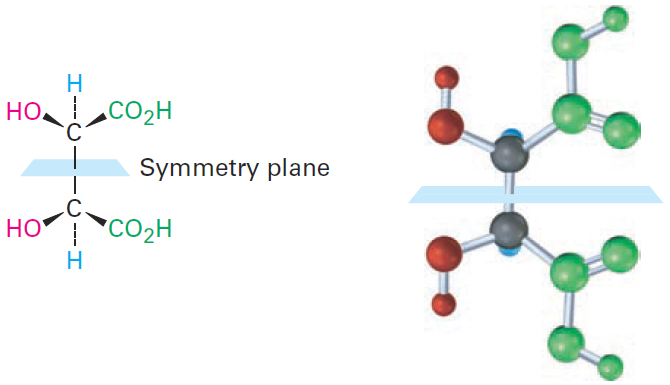
Figure 1-1 A symmetry plane through the C2–C3 bond of mesotartaric acid makes the molecule achiral.
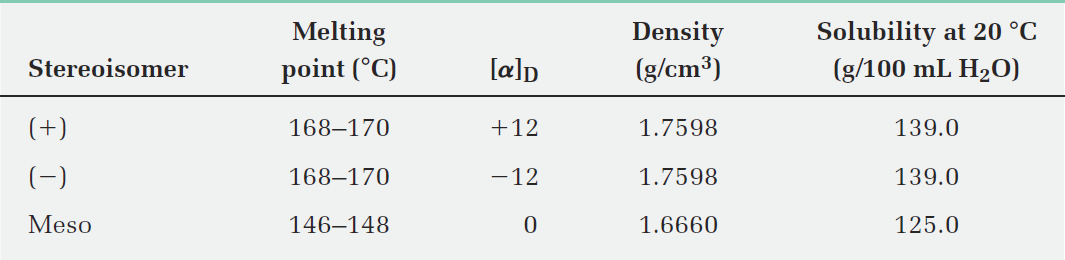
Some physical properties of the three stereoisomers are listed in Table 1. The (1)- and (2)-tartaric acids have identical melting points, solubilities, and densities, but they differ in the sign of their rotation of plane-polarized light. The meso isomer, by contrast, is diastereomeric with the (+) and (-) forms. It has no mirror-image relationship to (+)- and (-)-tartaric acids, is a different compound altogether, and has different physical properties.
Table 1:Some Properties of the Stereoisomers of Tartaric Acid



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|