

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Electronegativity values
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
p36
30-5-2016
991
Electronegativity values
In a homonuclear diatomic molecule X2, the electron density in the region between the nuclei is symmetrical; each X nucleus has the same effective nuclear charge. On the other hand, the disposition of electron density in the region between the two nuclei of a heteronuclear diatomic molecule X_Ymay be asymmetrical. If the effective nuclear charge of Y is greater than that of X, the pair of electrons in the X_Y covalent bond will be drawn towards Y and away from X.
Pauling electronegativity values, xP
In the early 1930s, Linus Pauling established the concept of electronegativity which he defined as ‘the power of an atom in a molecule to attract electrons to itself ’ (the electron withdrawing power of an atom). The symbol for electronegativity is x but we distinguish between different electronegativity scales by use of a superscript, e.g. x P for Pauling. Pauling first developed the idea in response to the observation that experimentally determined bond dissociation enthalpy values for heteronuclear bonds were often at variance with those obtained by simple additivity rules. Equation 1.1 shows the relationship between the bond dissociation enthalpy, D, of the homonuclear diatomic X2 and the enthalpy change of atomization, ΔaHo, of X.

 (1.1)
(1.1)
Effectively, this partitions bond enthalpy into a contribution made by each atom and, in this case, the contributions are equal.
In equation 1.2, we apply the same type of additivity to the bond in the heteronuclear diatomic XY. Estimates obtained for D(X–Y) using this method sometimes agree quite well with experimental data (e.g. ClBr and ClI), but often differ significantly (e.g. HF and HCl) as worked example below

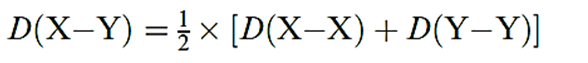 (1.2)
(1.2)
Worked example Bond enthalpy additivity
Given that D(H–H) and D(F–F) in H2 and F2 are 436 and 158 kJ mol-1, estimate the bond dissociation enthalpy of HF using a simple additivity rule. Compare the answer with the experimental value of 570 kJ mol-1.
Assume that we may transfer the contribution made to D(H–H) by an H atom to D(H–F), and similarly for F.


 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















