
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Nuclear Constituents
المؤلف:
Roger J Blin-Stoyle, FRS
المصدر:
Physics of Particles, Matter and the Universe
الجزء والصفحة:
p 123
24-5-2016
2527
Nuclear Constituents
The work of Rutherford, Geiger and Marsden established the existence of atomic nuclei and that they are very small (of the order 10-14m for gold atoms). Nuclei have positive electric charge to hold the negatively charged atomic electrons in their ‘orbits’ and, since atoms are electrically neutral, this nuclear charge must exactly balance the total negative charge carried by the surrounding electrons. If the number of electrons in an atom is denoted by Z (the atomic number) and the electron charge by -e it follows that the nuclear charge must be +Ze. Most of the mass of an atom is in its nucleus, the mass of the lightest (hydrogen) being around 1840 times the mass of an electron. The masses of heavier nuclei turn out to be roughly equal to integer multiples of the mass of the hydrogen nucleus. This integer is known as the muss number and is denoted by A. It takes the value 1 (for hydrogen), 4 for helium and so on through to around 260 for the heaviest elements. A is usually of the order two or more times the value of Z. These observations suggested first of all that atomic nuclei consist of two types of component-hydrogen nuclei (now called protons denoted by p) and electrons (denoted by e-). To have the mass roughly right there would need to be A protons and since the charge of each proton is +e there would need to be sufficient electrons (charge -e) in the nucleus to reduce the total proton charge (+Ae) to +Ze. However, simple as this idea is, it cannot work since, confining an electron to a nucleus of size 10-14m means that the uncertainty in its position is very small. In turn, the quantum mechanical uncertainty relation implies that the uncertainty in its momentum (and therefore its kinetic energy) is very large. It turns out that this kinetic energy is so large that the electrical attraction between the positively charged protons and the negatively charged electrons is far too weak to confine the electrons in the nucleus; the quantum mechanical agitation due to their confinement means that they would simply break out of the nucleus. The solution to this problem finally emerged in 1932 when an experiment by Chadwick established the existence of a new electrically neutral particle having virtually the same mass as a proton. This particle is known as a neutron (denoted by n) and an atomic nucleus can then be regarded as consituted from Z protons (to get the charge +Ze right) and N = A - Z neutrons (to get the mass right; Z + N = A). The idea that such particles might exist had been mooted by Rutherford some years earlier. Chadwick established their existence in an experiment in which alpha particles from a radioactive source bombarded a piece of beryllium. It was already known that this led to the emission of some form of very penetrating electrically neutral radiation. Chadwick studied this radiation by allowing it to fall on paraffin wax (a hydrogen rich substance) from which it knocked out hydrogen nuclei (i.e. protons) as shown in figure 1.1. These protons were then detected in what is known as an ionization chamber. The latter is a chamber containing gas and two electrodes. When a proton enters the chamber it knocks electrons out of the gas atoms leaving positively charged ions. An electric potential difference between the electrodes then results in a burst of electric current in an external circuit due to the presence of these charged ions, so indicating the arrival of a proton. By studying this process Chadwick established that the penetrating radiation that
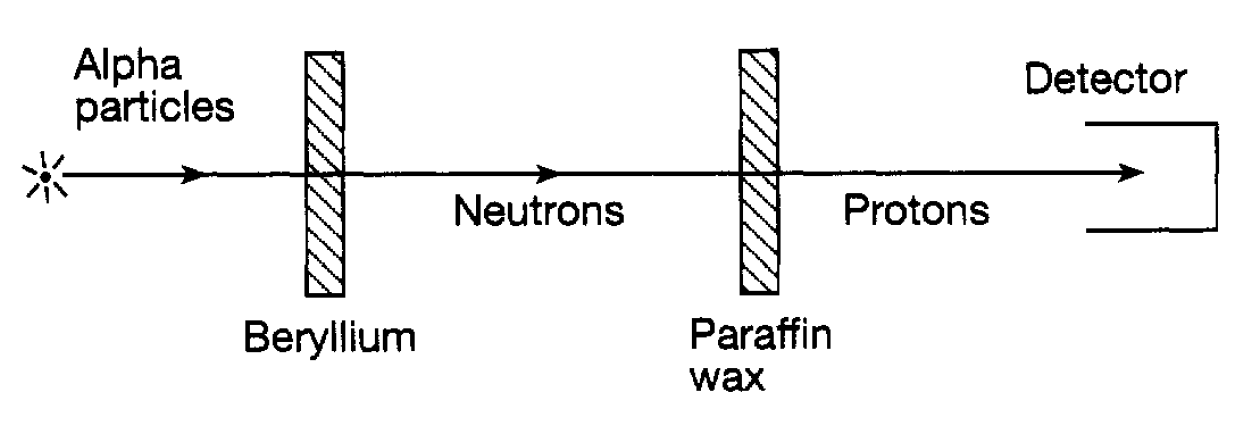
Figure 1.1: The Chadwick neutron experiment.
unleashed the protons was indeed composed of particles (neutrons) having a mass very close to that of protons. If the nucleus consists of neutrons and protons it follows that, for a particular chemical element defined by the number of electrons in the atom the mass of the nucleus can take various values. For an atom having Z electrons the nucleus must contain Z protons but the number of neutrons could vary. In practice this variation is limited and there are only a vew possibilities which lead to a stable or fairly stable structure for the nucleus. The atoms containing these different possible nuclei have identical chemical properties since they have the same number of electrons. They are referred to as isotopes and those that are only ‘fairly’ stable are radioactive. The foregoing description of atomic nuclei is straightforward and satisfying. The nucleus simply consists of an assembly of Z protons and N neutrons, the two types of particle being referred to collectively as nucleons. A particular nucleus X (say) is defined by the two numbers A and Z and denoted symbolically by 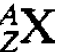 . For example
. For example  and
and  represent stable carbon isotopes,
represent stable carbon isotopes, 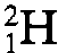 represents what is known as the heavy hydrogen nucleus and so on. However this description has a profound implication. There must be a very strong attractive force holding the nucleons together in the nucleus, not least because the electrical repulsion between the component protons, which are very close together, has to be withstood. We shall consider the nature and origin of this nuclear force which is much stronger than the electric force and which is one example, of what is known as the strong interaction, but before discussing this force a few of the general properties of nuclei for which the force must account will be described.
represents what is known as the heavy hydrogen nucleus and so on. However this description has a profound implication. There must be a very strong attractive force holding the nucleons together in the nucleus, not least because the electrical repulsion between the component protons, which are very close together, has to be withstood. We shall consider the nature and origin of this nuclear force which is much stronger than the electric force and which is one example, of what is known as the strong interaction, but before discussing this force a few of the general properties of nuclei for which the force must account will be described.
 الاكثر قراءة في مواضيع عامة في الفيزياء النووية
الاكثر قراءة في مواضيع عامة في الفيزياء النووية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















