


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 8-5-2016
Date: 18-5-2016
Date: 1-12-2015
|
Hepatitis B Virus
Hepatitis B virus (HBV) is one of the hepadnaviruses and causes acute, self-limited or persistent infection that can potentially be passed vertically from carrier mother to infant, or horizontally by the percutaneous and transmucosal exchange of infected blood and body fluids. Persistent infection by HBV is often associated with chronic liver disease that can lead to the development of hepatocellular carcinoma (HCC). The neonatal transmission route is thought to be an important mechanism for HBV persistence because of the immunological immaturity of the infected host. The concomitant infection is usually self-limited, with clearance of virus particles from the liver and blood and the development of lasting immunity to reinfection.
HBV is a spherical particle, 42 nm in diameter (Fig. 1). The genome of HBV is a circular DNA duplex of 3.2 kbp that replicates by reverse transcription and Rous Sarcoma Virus (RSV)) of a pre-genome RNA and contains a single-stranded gap region of variable length in one strand. DNA replication and transcription of viral genes and the mechanism of virion assembly within infected hepatocytes have been unraveled so some extent, but less is known about the virus–cell interactions that control virus attachment, uncoating, and entry into susceptible cells, as well as the integration of HBV DNA into the host cell genome. Although viral sequences implicated in the interaction of the virion with the cellular membrane are located in a variable region of the amino-terminal part of the pre-S1 domain, the cellular receptor for HBV has not yet been fully characterized.

Figure 1. Schematic diagram of a hepatitis B virus particle.
Nucleotide sequences and those amino acid sequences deduced from HBV DNA from different virus subtypes (adw, adr, ayw, ayr) have revealed a compact, overlapping coding organization of the genome, where the entire genome is coding (Fig. 2). Four open reading frames (ORFs) are localized on one viral strand in the same transcriptional polarity. Two of them, the C and S regions, specify structural proteins: the 183-residue nucleocapsid protein HBcAg and the 226-residue envelope major protein HBsAg, respectively. The smallest ORF, X, codes for a trans-acting 154-residues regulatory protein that interacts with various cellular proteins and may play a major role in hepatic cell transformation. Overlapping these coding regions, the largest ORF, P, encodes a viral polymerase of 843 residues, which is necessary for viral replication and has activities of DNA polymerase, reverse transcriptase, plus ribonuclease H. This overlapping of the P region is a characteristic feature and, with its unusual overlapping of coding and regulatory sequences (such as promoters, two specific enhancers, and a poly A termination signal), makes this virus unique. In addition to the central homologous region, two strong homology blocks in the amino- and carboxy-terminal parts of the X protein might correspond to a conservative selective pressure for functional activity of the X as a trans-regulator, and of the viral ribonuclease H encoded by C-terminal sequences of P, which overlap with the 5′ end of the X protein.
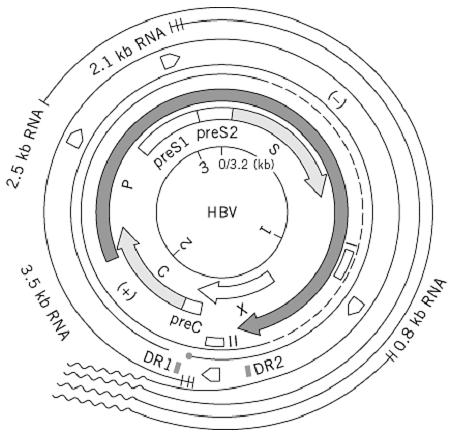
Figure 2. Genetic organization and transcription map of the hepatitis B virus genome. The HBV DNA was derived from virus of the adr subtype, with a nucleotide sequence of 3215 bp. The positions of promoters and two enhancers are indicated by and ƒ, respectively. The four different transcripts are indicated with a poly(A) tail (~). The terminal protein domain of polymerase (•) is attached to the 5′ end of the minus strand (—). DR1 and DR2 represent 11-bp direct repeats.
Another important characteristic of HBV is a high mutation rate due to the lack of a proofreading function of the reverse transcriptase that replicates its genome. This may increase the production of mutated variants during persistent infection, which enables the virus to escape from the host immune response or enables a growth advantage of mutant viral genomes. Substantial nucleotide and amino acid sequence heterogeneity in different isolates of HBV, plus specific mutations in the HBV genome in individual patients, have been observed, and partially deleted HBV genomes have also been observed in human hepatocellular carcinoma. However, there is no experimental evidence that these defective HBV genomes might harbor oncogenic potential, nor that any particular HBV subtype might be more oncogenic than others. Comparison of the HBV genome with those of animal hepadnaviruses reveals extensive homologies among mammalian hepadnaviruses (WHV, GSHV), which share a basically identical genomic organization.
The antibody response to HBV envelope antigen (HBsAg) is well characterized to be T-cell-dependent and plays an important role in clearance of the circulating virus and preventing their attachment and uptake by susceptible hosts. The role of the antibody response to the nucleocapsid ) HBcAg and its derivative in serum, HBeAg) and nonstructural proteins (X and P) in HBV pathogenesis is less clear yet. Persistent infection of HBV is mainly caused by a weak antiviral immune response that cannot kill or cure all of the infected cells. Alternatively, chronic hepatitis may be caused by an inappropriate T-cell response that can kill but cannot cure the infected hepatocytes without enough production of cytokines to clear the infection.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|