
The bonding in He2, Li2 and Be2
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
INORGANIC CHEMISTRY
المصدر:
INORGANIC CHEMISTRY
 الجزء والصفحة:
p31
الجزء والصفحة:
p31
 14-4-2016
14-4-2016
 2745
2745
The bonding in He2, Li2 and Be2
Molecular orbital theory can be applied to any homonuclear diatomic molecule, but as more valence atomic orbitals become available, the MO diagram becomes more complex.
Treatments of the bonding in He2, Li2 and Be2 are similar to that for H2. In practice, He does not form He2, and the construction of an MO diagram for He2 is a useful exercise because it rationalizes this observation. Figure 1.2a shows that when the two 1s atomic orbitals of two He atoms interact, σ and σ* MOs are formed as in H2. However, each He atom contributes two electrons, meaning that in He2, both the bonding and antibonding MOs are fully occupied. The bond order (equation 1.1) is zero and so the MO picture of He2 is consistent with its non-existence.
Using the same notation as for H2, the ground state electronic configuration of He2 is σg (1s)2 σu σ*g (1s)2 σ*u.
The ground state electronic configuration of Li (Z = 3) is 1s2 2s1 and when two Li atoms combine, orbital overlap occurs efficiently between the 1s atomic orbitals and between the 2s atomic orbitals. To a first approximation we can ignore 1s–2s overlap since the 1s and 2s orbital energies are
poorly matched. An approximate orbital interaction diagram for the formation of Li2 is given in Figure 1.2b.
Each Li atom provides three electrons, and the six electrons in Li2 occupy the lowest energy MOs to give a ground state electronic configuration of σg (1s)2 σ*u (1s)2 σg (2s)2. Effectively, we could ignore the interaction between the core 1s atomic orbitals since the net bonding is determined by the interaction between the valence atomic orbitals, and a simpler, but informative, electronic ground state is σg (2s)2.
Figure 1.2b also shows that Li2 is predicted to be diamagnetic in keeping with experimental data. By applying equation 1.1 , we see that MO theory gives a bond order in Li2 of one. Note that the terminology ‘core and valence orbitals’ is equivalent to that for ‘core and valence electrons’.

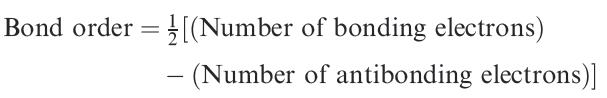 (1.1)
(1.1)
Like Li, Be has available 1s and 2s atomic orbitals for bonding; these atomic orbitals constitute the basis set of orbitals. An orbital interaction diagram similar to that for Li2 (Figure 1.2b) is appropriate. The difference between Li2 and Be2 is that Be2 has two more electrons than Li2 and these occupy the σ*(2s) MO. The predicted bond order in Be2 is thus zero. In practice, this prediction is essentially fulfilled, although there is evidence for an extremely unstable Be2 species with bond length 245pm and bond energy 10 kJ mol-1.
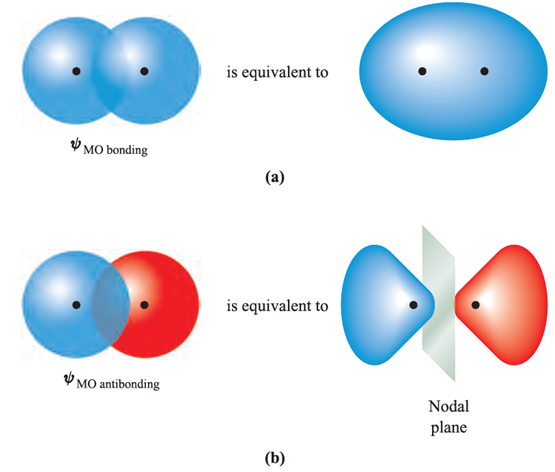
Fig. 1.1 Schematic representations of (a) the bonding and (b) the antibonding molecular orbitals in the H2 molecule. The H nuclei are represented by black dots. The red orbital lobes could equally well be marked with a sign, and the blue lobes with a + sign to indicate the sign of the wavefunction.

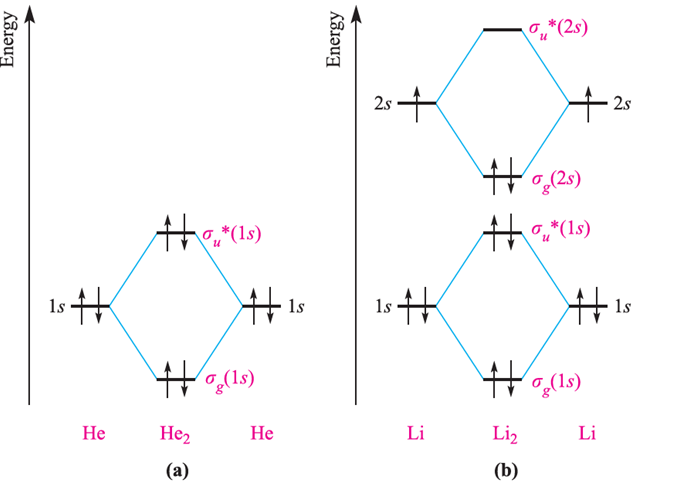
Fig. 1.2 Orbital interaction diagrams for the formation of (a) He2 from two He atoms and (b) Li2 from two Li atoms.
A basis set of orbitals is composed of those which are available for orbital interactions. In each of Li2 and Be2, it is unnecessary to include the core (1s) atomic orbitals in order to obtain a useful bonding picture. This is true more generally, and throughout this book, MO treatments of bonding focus only on the interactions between the valence orbitals of the atoms concerned.
 الاكثر قراءة في نظريات التآصر الكيميائي
الاكثر قراءة في نظريات التآصر الكيميائي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


