

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
General Approach to Infectious Diseases
المؤلف:
Gallagher ,J.C. and MacDougall ,c.
المصدر:
Antibiotics Simplified
الجزء والصفحة:
24-3-2016
1688
General Approach to Infectious Diseases
The pharmacotherapy of infectious diseases con-fuses many clinicians, but the approach to the patient with an infection is relatively simple and consistent. Understanding this approach is the first step in developing a useful expertise in infectious diseases and antibiotic use. A note: technically the term antibiotic refers only to a subset of antibacterial drugs that are natural products. The terms anti-infective and antimicrobial encompass antibacterial, antifungal, antiviral, and antiparasitic drugs. However, because antibiotic is the more commonly used term, we will use it to refer to antimicrobials in general or antibacterials specifically.
Prophylactic Therapy
The use of antimicrobial chemotherapy—that is, the treatment of microorganisms with chemical agents—falls into one of three general categories: prophylaxis, empiric use, and definitive therapy. Prophylaxis is treatment given to prevent an infection that has not yet developed. Use of prophylactic therapy should be limited to patients at high risk of developing an infection, such as those on immuno-suppressive therapy, those with cancer, or patients who are having surgery. These patients have weakened natural defenses that render them susceptible to infection. Because the likelihood of infection by some types of organisms in these patients is high and the consequences of infection are dire, we ad-minister antimicrobial drugs to prevent infections from occurring. However, the world is not sterile and breakthrough infections do occur. The key to understanding antimicrobial prophylaxis is to re-member that patients who receive it do not have an infection, but they are at risk for one.
Empiric Therapy
Unlike prophylactic therapy, empiric therapy is given to patients who have a proven or suspected infection, but the responsible organism(s) has or have not yet been identified. It is the type of therapy most often initiated in both outpatient and in-patient settings. After the clinician assesses the likelihood of an infection based on physical exam, laboratory findings, and other signs and symptoms, he or she will usually collect samples for culture and Gram staining. For most types of cultures, the Gram stain is performed relatively quickly. In the Gram stain, details about the site of presumed infection are revealed, such as the presence of organ-isms and white blood cells (WBCs), morphology of the organisms present (e.g., Gram-positive cocci in clusters), and the nature of the sample itself, which in some cases indicates if the sample is adequate. The process of culturing the sample begins around the time that the clinician performs the Gram stain. After a day or so, biochemical testing will reveal the identification of the organism, and eventually the organism will be tested for its susceptibility to various antibiotics.
However, this process takes several days, so empiric therapy is generally initiated before the clinician knows the exact identification and susceptibilities of the causative organism. Empiric therapy is our best guess of which antimicrobial agent or agents will be most active against the likely cause of infection. Sometimes we are right, and some-times we are wrong. Keep in mind that empiric therapy should not be directed against every known organism in nature—just those most likely to cause the infection in question. In other words, broad-spectrum antibiotics are not a substitute for rational thought!
Definitive Therapy
After culture and sensitivity results are known, the definitive therapy phase of treatment can begin. Unlike empiric therapy, with definitive therapy we know on what organisms to base our treatment and which drugs should work against them. At this phase it is prudent to choose antimicrobial agents that are safe, effective, narrow in spectrum, and cost effective. This helps us avoid unneeded toxicity, treatment failures, and the possible emergence of antimicrobial resistance, and it also helps man-age costs. In general, moving from empiric to definitive therapy involves decreasing coverage, because we do not need to target organisms that are not causing infection in our patient. In fact, giving overly broad-spectrum antibiotics can lead to the development of superinfections, infections caused by organisms resistant to the antibiotics in use that occur during therapy.
The clinician who is treating an infected patient should always strive to make the transition to definitive therapy. Although it seems obvious, this does not always occur. If the patient improves on the first antibiotic, clinicians may be reluctant to transition to more narrow-spectrum therapy. Also, some infections may resolve with empiric therapy before culture results would even be available, as happens with uncomplicated urinary tract infections (UTIs). In other cases, cultures may not be obtained or may be negative in spite of strong signs that the patient has an infection (e.g., clinical symptoms, fever, increased WBC count). In most situations it is important that clinicians continuously consider the need to transition to definitive therapy. Overly broad-spectrum therapy has consequences, and the next infection is likely to be harder to treat. Keep in mind the general pathway for the treatment of infectious diseases shown in figure 1 .
Examples of Therapy
Here are a few examples of each type of therapy:
Prophylaxis
• Trimethoprim/sulfamethoxazole (TMP/SMX) to prevent Pneumocystis jirovecii (formerly carinii) pneumonia in a patient on cyclosporine and prednisone after a liver transplant
• Azithromycin to prevent Mycobacterium Avium intracellularae (MAI or MAC) in an advanced HIV patient
• Cefazolin given before surgery to prevent a staphylococcal skin infection of the surgical site
Empiric Therapy
• Levofloxacin initiated for a patient with presumed community-acquired pneumonia
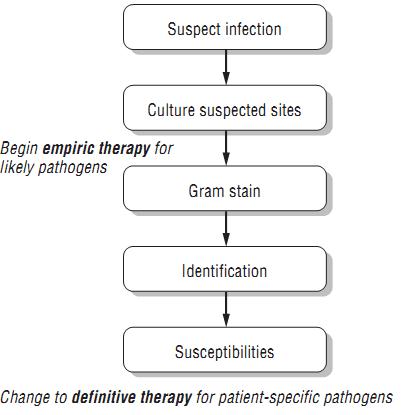
Figure 1: General Approach to Infectious Diseases
• Ceftriaxone given for the treatment of suspected pyelonephritis.
• Voriconazole initiated for a neutropenic bone marrow transplant patient with shortness of breath and a radiograph suggestive of pulmonary aspergillosis .
• Vancomycin, tobramycin, and meropenem for a patient with probable hospital-acquired pneumonia in the intensive care unit .
Definitive Therapy
• Transitioning from piperacillin/tazobactam to ampicillin in a patient with a wound infection caused by Enterococcus faecalis, which is susceptible to both drugs.
• Discontinuing ceftriaxone and initiating ciprofloxacin for a patient with a UTI caused by Klebsiella pneumoniae that is resistant to ceftriaxone but susceptible to ciprofloxacin
• Stopping caspofungin and initiating fluconazole for a patient with Candida in a blood isolate when the species is identified as Candida albicans (which is reliably susceptible to fluconazole)
• Narrowing therapy from vancomycin, ciprofloxacin, and imipenem/cilastatin to vancomycin alone for a patient with hospital-acquired pneumonia whose deep respiratory culture grew only methicillin-resistant Staphylococcus aureus (MRSA) that is susceptible to vancomycin .
Case Study
Here is an example of treating a patient with an infection by the above pathway:
TR is a 63-year-old man with a history of diabetes, hypertension, and coronary artery disease who comes to the hospital complaining of pain, redness, and swelling around a wound on his foot. Close inspection reveals that he has an infected diabetic foot ulcer. He is admitted to the hospital (Day 1). The clinician per-forms surgical debridement that evening and sends cultures from the wound during surgery as well as blood cultures. The clinician initiates empiric therapy with vancomycin and piperacillin/tazobactam.
On Day 2, Gram stain results from the wound are available. There are many WBCs with many Gram-positive cocci but no Gram-negative rods (GNRs), so the clinician discontinues piperacillin/tazobactam. Blood cultures do not grow any organisms.
The following day (Day 3), culture results from the wound reveal many Staphylococcus aureus. Because vancomycin is usually effective against this organism, its use is continued.
On Day 4, susceptibility results from the wound culture return. The S. aureus is found to be susceptible to methicillin, oxacillin, cefazolin, piperacillin/ tazobactam, clindamycin, TMP/SMX, and vancomycin. It is resistant to penicillin, ampicillin, tetracycline, and levofloxacin. Because the isolate from TR’s wound is methicillin-sensitive Staphylococcus aureus (MSSA), the clinician discontinues vancomycin and initiates definitive therapy with oxacillin.
Note how in TR’s case we began empiric therapy with a broad-spectrum regimen of vancomycin and piperacillin/tazobactam to cover the Gram-positive and Gram-negative aerobes and anaerobes that tend to cause diabetic foot infections but narrowed that therapy gradually as Gram stain and culture data returned. Eventually we were able to choose a highly effective, narrow-spectrum, inexpensive, and safe choice of definitive therapy that was driven by micro-biology results. Both vancomycin and piperacillin/ tazobactam were active against TR’s Staphylococcus aureus as well, but both are broader in spectrum than oxacillin and represent less-ideal therapy choices.
References
Gallagher ,J.C. and MacDougall ,c. (2012). Antibiotics Simplified. Second Edition. Jones & Bartlett Learning, LLC.
 الاكثر قراءة في مواضيع عامة في المضادات الميكروبية
الاكثر قراءة في مواضيع عامة في المضادات الميكروبية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















