


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 7-3-2016
Date: 17-3-2016
Date: 3-3-2016
|
Cronobacter
1. Introduction
Cronobacter ( Enterobacter sakazakii) causes a foodborne disease classified by the International Commission on Microbiological Specifications for Foods (ICMSF, 2002) in Risk Group IB: “diseases of severe hazard for restricted population; life threatening or resulting in substantial chronic sequelae or presenting effects of long duration”. The FAO/WHO (Food and Agriculture Organization/World Health Organization) expert meetings have identified all infants (less than 12 months of age) as the population at particular risk for Cronobacter infections. Among this group, those at greatest risk are neonates (less than 28 days), particularly preterm, low-birth-weight (less than 2500 g), and immunocompromised infants, and those less than two months of age.
Infants of HIV-positive mothers are also at risk, because they may specifically require infant formula and may be more susceptible to infection (Codex Alimentarius/CAC/RCP 66, 2008 revision 1 2009).
2. Taxonomy
The information below is from Iversen et al. (2007) and Iversen et al. (2008). Cronobacter is a member of the family Enterobactericeae and until 1980 was designated as a yellow pigmented variant of Enterobacter cloacae. In 1980 it was defined as the new species [Enterobacter sakazakii] by Farmer et al (1980). DNA-DNA hybridization gave no clear generic assignment for [E. sakazakii] as it was shown to be 53–54% related to species in two different genera, Enterobacter and Citrobacter. However the species was placed in Enterobacter as it appeared phenotypically and genotypically closer to E. cloacae than to C. freundii, the type species of these genera. Iversen et al. (2007) proposed the subdivision of the species [E. sakazakii] in new species allocated in the new genus Cronobacter. Iversen et al. (2008) reported officially the taxonomy of the new genus and the new species.
- Cronobacter Iversen et al. 2008, gen. nov.
The members of the genus Cronobacter are Gram-negative rods, oxidase-negative, catalase-positive, and facultative anaerobic. Generally motile, they reduce nitrate, utilize citrate, hydrolyze aesculin and arginine and test positive for L-ornithine decarboxylation. Acid is produced from D-glucose, sucrose, raffinose, melibiose, cellobiose, D-mannitol, D-mannose, L-rhamnose, L-arabinose, D-xylose, trehalose, galacturonate and maltose. Generally positive for acetoin production (Voges-Proskauer test) and negative for the methyl red test, indicating 2,3-bu-tanediol rather than mixed acid fermentation. Negative reactions include hydrogen sulphide (H2S) production, urea hydrolysis, lysine decarboxylation and β-D-glucuronidase activity. The production of indole is variable.
The temperature range for growth is between 6°C and 45°C (in brain heart infusion broth) and the pH range is between pH 5.0 and 10.0, with no growth below pH 4.5. Able to grow in up to 7% (w/v) NaCl but not in 10%.The differentiation among the new genus Cronobacter and some other genera of the Enterobacteriaceae family is shown in the Table 1. The differentiation among the new Cronobacter species is shown in the Table 2.
3. Epidemiology
The information below is from Codex Alimentarius/CAC/RCP 66 (2008 revision 1 2009).
According to the FAO/WHO (2008) experts all the six species of Cronobacter should be considered patho-genic. The incidence of infections in infants is low but have been documented as sporadic cases or as outbreaks with severe consequences.
Table.1 Biochemical characteristics used to differentiate the new genus Cronobacter from some other genera of the Enterobacteriaceae family (Iversen et al., 2007).

Manifestations may occur as meningitis and bacteraemia, with variable fatality rates which may be as high as 50 percent (reported in at least one outbreak). Surviving infants have sequelae such as retardation and other neurological conditions.
Outbreaks of Cronobacter species infections have been linked to powdered formulae, especially cases occurred in neonatal intensive care setting. Low number of Cronobacter cells is present in a proportion of powdered formulae. The microorganism has also been detected in other types of food, but only powdered formulae has been linked to outbreaks of disease. For infants at greatest risk in neonatal intensive care settings, commercially sterile liquid infant formula should be used. If a non-commercially sterile feeding option is chosen, an effective decontamination procedure should be used.
4. Codex Alimentarius microbiological criteria for Cronobacter spp. in powdered infant formulae
The Codex Alimentarius established the “Code of Hygienic Practice for Powdered Formulae for Infants and Young Children” (Codex Alimentarius/CAC/RCP 66, 2008 revision 1 2009), to provide practical guidance, recommendations, sampling plans, and microbiological criteria on the hygienic manufacture of powdered infant formulae.
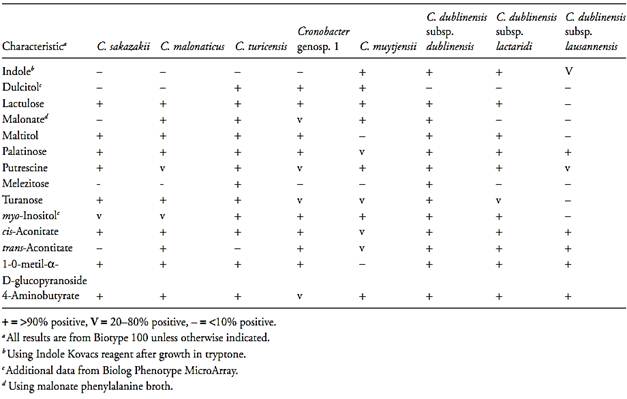
Table.2 Biochemical tests used to differentiate the species and subspecies of the genus Cronobacter (Iversen et al., 2008)
• Infant formulae and formulae for special medical purposes intended for infants (not more than 12 months of age), which serve as the sole source of nutrition.
• Follow-up formulae which are used in combination with other foods as part of the weaning diet of older infants and young children (from the age of more than 12 months up to the age of three years).
• Powdered formulae for special medical purposes for infants and young children, intended to partially replace or supplement breast milk, infant formulae or follow-up formulae.
• Human milk fortifiers used to supplement breast milk. The sampling plans and microbiological criteria for finished product include Salmonella, Cronobacter, Enterobacteriaceae, and mesophilic aerobic bacteria (Table.3).
Table.3 Microbiological criteria applied by the Codex Alimentarius to powdered infant formulae (finished product) (Codex Alimentarius/CAC/RCP 66, 2008 revision 1 2009).

References
Chap, J., Jackson, P., Siqueira, R., Gaspar, N., Quintas, C., Park, J., Osaili, T., Shaker, R., Jaradat, Z., Hartantyo, S.H.P., Abdullah Sani, N., Estuningsih, S. & Forsythe, S.J. (2009). International survey of Cronobacter sakazakii and other Cronobacter spp. in follow up formulas and infant foods. International Journal of Food Microbiology, 136(2), 185–188.
Codex Alimentarius (2009) CAC/RCP 66:2008 revision 1: 2009. Code of Hygienic Practice for Powdered Formulae for Infants and Young Children. [Online] Rome, Italy. Available from: http:// www.codexalimentarius.net/web/standard_list.jsp [Accessed 12th December 2011].
FAO/WHO (2008). Enterobacter sakazakii (Cronobacter spp.) in powdered follow-up formulae. [Online] Food and Agriculture Organization/World Health Organization. Meeting Report: Microbiological Risk Assessment Series 15. Available from: http://www.fao.org/ag/agn/agns/jemra/mra15_sakazaki.pdf [Accessed 12th December 2011].
Farmer III, J.J., Asbury, M.A., Hickman, F. W., Brenner, D. J. & The Enterobacteriaceae Study Group (1980) Enterobacter sakazakii: a new species of “Enterobacteriaceae” isolated from clinical specimens. International Journal of Systematic Bacteriology, 30(3), 569–584.
ICMSF (International Commission on Microbiological Specifications for Foods) (ed.) (2002) Microorganisms in Foods 7. Micro-biological Testing in Food Safety Management. New York, Kluwer Academic/Plenum Publishers.
International Organization for Standardization (2006) ISO 22964:2006. Milk and milk products – Detection of Enterobacter sakazakii. Geneva, ISO.
Iversen, C., Lehner, A., Mullane, N., Bidlas, E., Cleenwerck, I., Marugg, J., Fanning, S., Stephan, R. & Joosten, H. (2007) The taxonomy of Enterobacter sakazakii: proposal of a new genus Cronobacter gen. nov. and descriptions of Cronobacter sakazakii comb. nov. Cronobacter sakazakii subsp. sakazakii, comb. nov., Cronobacter sakazakii subsp. malonaticus subsp. nov., Cronobacter turicensis sp. nov., Cronobacter muytjensii sp. nov., Cronobacter dublinensis sp. nov. and Cronobacter genomospecies 1. BMC Evolutionary Biology, 7, 64.
Iversen, C., Mullane N., McCardell, B.,Tall, B.D., Lehner, A., Fan-ning, S., Stephan, R. & Joosten, H. (2008) Cronobacter gen. nov., a new genus to accommodate the biogroups of Enterobacter sakazakii, and proposal of Cronobacter sakazakii gen. nov., comb. nov., Cronobacter malonaticus sp. nov., Cronobacter turicensis sp. nov., Cronobacter muytjensii sp. nov., Cronobacter dublinensis sp. nov., Cronobacter genomospecies 1, and of three subspecies, Cronobacter dublinensis subsp. dublinensis subsp. nov., Cronobacter dublinensis subsp. lausannensis subsp. nov. and Cronobacter dublinensis subsp. lactaridi subsp. nov. International Journal of Systematic and Evolutionary Microbiology, 58, 1442–1447.



|
|
|
|
مخاطر خفية لمكون شائع في مشروبات الطاقة والمكملات الغذائية
|
|
|
|
|
|
|
"آبل" تشغّل نظامها الجديد للذكاء الاصطناعي على أجهزتها
|
|
|
|
|
|
|
قسم الشؤون الفكرية يصدر العدد الثاني والعشرين من سلسلة كتاب العميد
|
|
|