
Isotopes: Varying neutrons
 المؤلف:
John T. Moore, EdD
المؤلف:
John T. Moore, EdD
 المصدر:
Chemistry Essentials For Dummies
المصدر:
Chemistry Essentials For Dummies
 الجزء والصفحة:
p31
الجزء والصفحة:
p31
 15-3-2016
15-3-2016
 1962
1962
Isotopes: Varying neutrons
The atoms of a particular element can have an identical number of protons and electrons but varying numbers of neutrons. If they have different numbers of neutrons, then the atoms are called isotopes.
Hydrogen is a common element here on Earth. Hydrogen’s atomic number is 1 — its nucleus contains 1 proton. The hydrogen atom also has 1 electron. Because it has the same number of protons as electrons, the hydrogen atom is neutral (the positive and negative charges have canceled each other out).
Most of the hydrogen atoms on Earth contain no neutrons. You can use the symbolization in below Figure to represent hydrogen atoms that don’t contain neutrons, as shown Figure 1.a shows. However, approximately one hydrogen atom out of 6,000 contains a neutron in its nucleus.
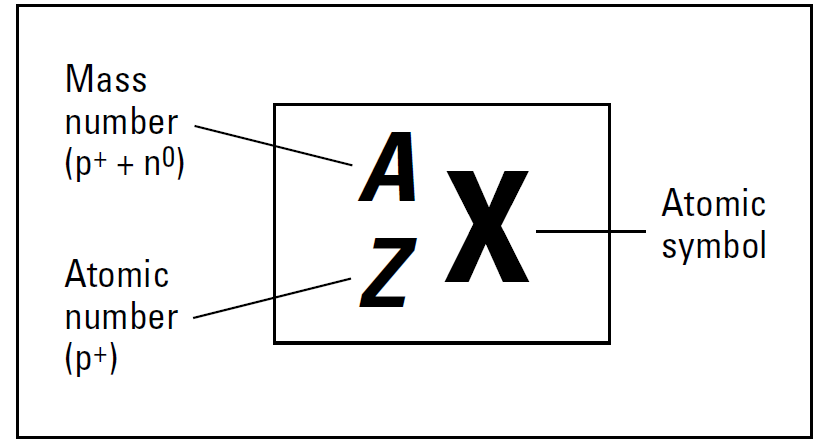
These atoms are still hydrogen, because they each have one proton; they simply have a neutron as well, which most hydrogen atoms lack. So these atoms are called isotopes. Figure 1.b shows an isotope of hydrogen, commonly called deuterium. It’s still hydrogen, because it contains only one proton, but it’s different from the hydrogen in Figure 1.a, because it also has one neutron. Because it contains one proton and one neutron, its mass number is 2 amu. There’s even an isotope of hydrogen containing two neutrons. This one’s called tritium, and it’s represented in Figure 1.c. Tritium is extremely rare, but it can easily be created.
Figure 1. also shows an alternative way of representing isotopes: Write the element symbol, a dash, and then the mass number.
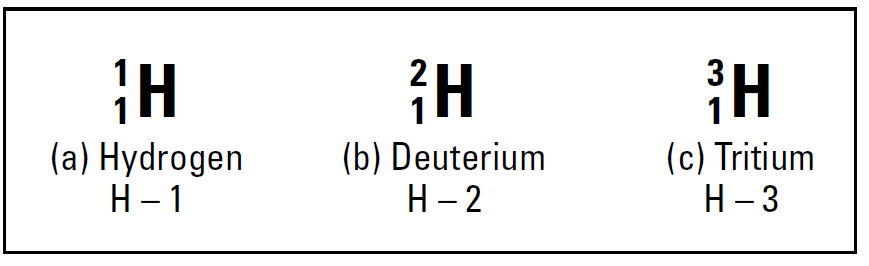
Figure1: The isotopes of hydrogen.
 الاكثر قراءة في مقالات متنوعة في علم الكيمياء
الاكثر قراءة في مقالات متنوعة في علم الكيمياء
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


