


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 2025-03-13
Date: 3-3-2016
Date: 21-3-2016
|
Enterococci
Introduction
The enterococci and fecal streptococci are the species of Enterococcus and Streptococcus associated with the gastrointestinal tracts of man and animals and traditionally used as indicator of fecal contamination. Until 1984 all the existing species which conformed to these characteristics were affiliated to the genus Streptococcus (known as “fecal streptococci” group) and have the Lancefield’s Group D antigen. Within the “ fecal streptococci” group there was a subgroup of species known as “ enterococci”, which differed from other fecal streptococci by their resistance to 0.4% of sodium azide, ability to grow in 6.5% of sodium chloride, at pH 9.6, and at 10°C and 45°C.
The serology classification of β-hemolytic streptococci was proposed by Lancefield (1933) to determine the group-specific carbohydrate antigens present in the cell wall, designated by letters of the alphabet (Leclerc et al., 1996). The group D was characteristically found among the species of the “ fecal streptococci” group, including the “ enterococci”.
The terms “enterococci”, “ fecal streptococci”, and “group D streptococci” have been used more ore less with the same meaning, to describe the intestinal streptococci (Leclerc et al., 1996). However, in 1984 the species of the subgroup “ enterococci” was separated from the genus Streptococcus and transferred to the new genus Enterococcus ( Enterococcus faecalis and Enterococcus faecium by Schleifer and Kilpper-Bälz, 1984 and Enterococcus avium; Enterococcus casselipavus; Enterococcus durans and Enterococcus gallinarum by Collins et al., 1984). Later several other species was incorporated to the genus but not all are of intestinal origin, not all have the Lancefield’s group D antigen and not all grow in 0.4% of azide, 6.5% of sodium chloride, at pH 9.6, and at 10°C and 45°C. In consequence, the term enterococci nowadays represent all the members of the genus Enterococcus, which are a ubiquity collection of species present not only in the intestine of the man and other animals, but also in fresh water, sea water, soil and plants. The origin of the species which compound the genus is presented in Table 1.
The species of intestinal streptococci maintained in the genus Streptococcus after the transference of the “enterococci” to the genus Enterococcus were S. bovis and S. equinus, both listed in the 1st edition of the Bergey’s Manual of Systematic Bacteriology (Hardie, 1986). Successive studies have resulted in the gradual subdivision of the S. bovis and S. equinus strains into additional species (Whiley and Hardie, 2009). This group of species is referred as the “ bovis group” or the “S. bovis/S. equinus complex” or the “ S. bovis/S. equinus group”. Based on DNA-DNA hybridization studies the type strains of S. bovis and S. equinus was placed in the same similarity group and these two names was recognized as subjective synonyms. Under the rules of the International Code of Nomenclature of Bacteria S. equinus epithet has priority. In 2011 the nomenclature of the species included within the “ bovis group” are S. equinus, S. alactolyticus, S. gallolyticus (subdivided into the subspecies S.gallolyticus subsp. gallolyticus, S.gallolyticus subsp. macedonicus and S.gallolyticus subsp. pasteurianus) and S. infantarius (Whiley and Hardie, 2009, DSMZ, 2011, Euzéby, 2011). The Lancefield’s group D antigen is found in all species of the “ bovis group” (if not in all strains, at least in some strains of each species). In addition to the “ bovis group”, three new species of Streptococcus isolated from the intestine of animals were described later: S. entericus (Vela et al., 2002), S. henryi and S. cabali (Milinovich et al., 2008). The group D antigen is present in S. entericus and S. henryi but not in S. cabali. The term “ fecal streptococci” nowadays rep-resent these species of the genus Streptococcus which are associated with the intestinal tract of humans and other animals. The nomenclature and main source of isolation of the intestinal streptococci species are presented in Table 2.
Table 1 Species of the genus Enterococcus and main source of isolation

(continued)
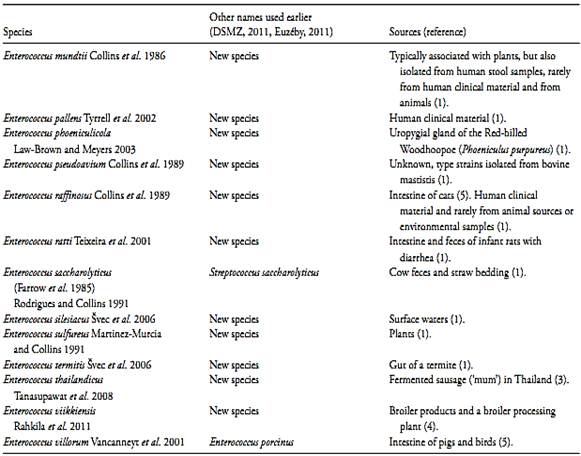
References: 1) Svec and Devriese (2009), 2) Leclerc et al. (1996), 3) Tanasupawat et al. 2008, 4) Rahkila et al. 2011, 5) Euzéby (2009).
1.1 Enterococci
Most species of Enterococcus are part of the intestinal flora of mammals, birds, and other animals. They are also isolated from foods, plants, soil and water.
Although commensal inhabitants of humans, they are increasingly isolated from a variety of nosocomial and other infections.
1.1.1 Species of intestinal origin
The species of intestinal origin are E. faecalis, E. faecium, E. durans, E. cecorum, E. hirae, E. villorum, E. raffinosus, E. canintestini, E. canis, and E. avium (Euzéby, 2009).
Euzéby (2009) summarized the species most common in the intestinal tract of humans and other animals:
• Humans - E. faecalis, E. faecium and, to a lesser extent, E. durans;
• Adult bovines - E. cecorum;
• Pigs - E. faecium, E. faecalis, E. hirae, E. cecorum and E. villorum;
• Poultry - E. durans, E. faecium and E. faecalis (in young animals) and E. cecorum in animals of more than 12 weeks);
• Calves - E. durans, E. faecalis and E. faecium;
• Young horses - E. durans;
• Cats - E. faecalis, E. faecium and, to a lesser extent, E. cecorum and E. raffinosus;
• Dogs - E. canintestini, E. canis, E. faecalis, E. faecium, and, to a lesser extent, E. hirae and E. avium.
Table 2 Species of the genus Streptococcus associated with the intestinal tract of humans and/or other animals.
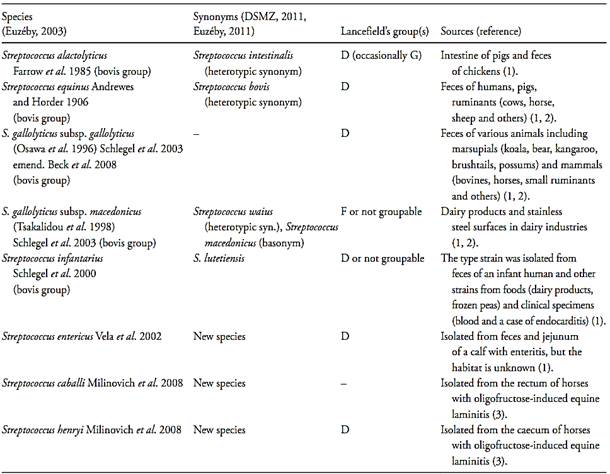
References: 1) Whiley and Hardie (2009), 2) Euzéby (2003), 3) Milinovich et al. (2008).
1.1.2 Species found in plants, soil and water
In the environment (plants, soil and water) the most common species are E. casseliflavus, E. haemoperoxidus, E. moraviensis, E. mundtii and E. sulfureus, but it can also be contaminated by other species like E. faecalis and E. faecium (Euzéby, 2009).
According to the information summarized by Svec and Devriese (2009), the enterococci are a temporary part of the microflora of plants, probably disseminated by insects. The soil is not naturally inhabited by enterococci but can be contaminated from animals, plants, wind or rain. In waters the presence of enterococci is considered an indication of fecal contamination and they have been used as indicators of distant contamination because of their long survival capacities.
Moore et al. (2007) evaluated the use of enterococci as indicator of fecal contamination in water samples from California and found that 42 to 54% of the Enterococcus isolated from urban runoff, bays and the ocean were E. casseliflavus and E. mundtii, plantassociated species. The remainder was fecal-associated species.
From sewage isolates 90% were E. faecalis and E. faecium as expected. False positives (non Enterococcus) ranged from 4 to 5% for urban runoff to 10 to 15% for bays and oceans. The distribution of species was similar for urban runoff, bays and oceans. Speciation could differentiate plant associated from fecal-associated species.
1.1.3 Species found in foods
The species most commonly found in foods are E. faecium and E. faecalis, although E. casseliflavus, E. durans, E. gallinarum, E. hermanniensis, E. hirae, E. italicus and E. mundtii are also isolated from foodstuffs (Euzéby (2009).
According to the information summarized by Svec and Devriese (2009), the enterococci are commonly found in foods, as spoilage agents or as adjuncts in the manufacturing of some types of cheese. E. faecium is the most common specie in cheese and combined products containing cheese and meat. E. faecalis is common in crustaceans.
E. faecium is the most common specie in meat products, followed by E. faecalis and by E. durans/E. hirae. E. gallinarum is found in products containing turkey meat.
E. hermanniensis is found in broiler meat and E. devriesei in vacuum-packaged charcoal-broiled river lampreys. E. faecalis and E. faecium are the most common species in frozen chicken carcasses, milk and milk products.
According to Cravem et al. (1997) the enterococci is widely used as indicator in water, but its use as indicator in foods has decreased significantly. Enterococci are more resistant to environmental factors than enterobacteria, and this is one of the main reasons why their use as indicator microorganisms has been criticized. They may survive under conditions that are lethal to enterobacteria, thus, their presence may have little or no relation at all with the presence of enteric pathogens.
On the other hand, their greater resistance may be useful to assess the effectiveness of disinfection procedures and programs of food processing plants or in evaluating the hygienic-sanitary quality of acid or frozen foods, in which coliforms or E. coli may not survive.
1.1.4 Biochemical characteristics of the genus Enterococcus
Genus description from Svec and Devriese (2009): The enterococci produce non-spore forming Gram-positive ovoid cells, occurring singly, in pairs or in short chains.
Most species are non-motile (strains of E. columbae, E. casseliflavus and E. gallinarum may be motile). Most species are not pigmented (E. gilvus, E. mundtii, E. pallens, E. sulfureus and some strains of E. casseliflavus and E. haemoperoxidus are yellow pigmented). Catalase is negative (some strains reveal pseudocatalase activity on media containing blood). Facultative anaerobic (certain species are CO2 dependent). Optimal growth temperature of most species is 35–37°C. Many but not all species are able to grow at 42°C and even at 45°C, and (slowly) at 10°C. Very resistant to drying. Chemoorganotrophs, the growth is generally dependent of complex nutrients. Homofermentative, the predominant end product of glucose fermentation is L(+) lactic acid. Certain characteristics are common to all described species, although rare exceptions may occur and certain tests results have not yet been reported in the lesser known species: resistance to 40% (v/v) bile, hydrolysis of esculin (β-glucosidase activity) and leucine arylamidase production positive. The characteristics traditionally considered to be typical for the genus do not apply to several of the more recently described species: Lancefield’s group D antigen, resistance to 0.4% of sodium azide or 6.5% of NaCl, growth at 10 and 45°C and production of pyrrolidonyl arylamidase (PYR).
The 2nd edition of Bergey’s Manual of Systematic Bacteriology (Svec and Devriese, 2009) divided the species into groups within the genus Enterococcus. Members of such groups exhibit similar phenotypic characteristics, and species separation can be problematic:
E. faecalis group: E. faecalis, E. caccae, E. hemoperoxidus, E. moraviensis, E. silesiacus and E. termitis. These species form similar dark red colonies with a metallic sheen on Slanetz-Bartley (m-Enterococcus) Agar. The growth at 10°C, in 6.5% of NaCl and the production of D antigen are positive. E faecalis is usually nonhemolytic and produces pseudocatalase when cultivated on blood containing agar media. Strains survive heating at 60°C for 30 min.
E. faecium group: E. faecium, E. durans, E. canis, E. hirae, E. mundtii, E. ratti, and E. villorum. These species are closely related and differentiation by biochemical tests is often unreliable. E. faecium grows at pH 9.6 and survives heating at 60°C for 30 min.
E. avium group: E. avium, E.devriesei, E.gilvus, E.maloduratus, E.pseudoavium and E. raffinosus. These species are mostly characterized by formation of small colonies with strong greening hemolysis on blood agar and weakly growth on Enterococcus selective media. They grow at 10°C, 45°C, and in 6.5% of NaCl and are typically adonitol and L-sorbose positive. The D anti-gen production may be negative.
E. gallinarum group: E. gallinarum and E. casseliflavus. They are typically motile, although nonmotile strains may be rarely found. The growth on Enterococcus selective media is poor and strongly enhanced by cultivation in a CO2 atmosphere (carboxyphilic).
E. italicus group: E. italicus and E. camelliae. They have low biochemical activity in comparison with the other enterococcal species.
13.1.2 Fecal streptococci
Most species of streptococci are commensal microorganisms in the oral cavity, upper respiratory tract and gastrointestinal tract of warm-blooded animals and birds.
In some instances they can cause infections (Whiley and Hardie, 2009). The 2nd edition of Bergey’s Manual of Systematic Bacteriology (Whiley and Hardie, 2009) divided the species into groups within the genus Streptococcus. The “pyogenic group” includes the species associated with pyogenic infections in man and/or other animals. The “mutans group” includes the species associated with dental plaque in man and several other animals. The “anginosus group” includes the species associated with purulent infections at oral and non-oral sites, isolated from the oral cavity, upper respiratory tract, intestinal tract and urogenital tract. The “mitis group” includes the species mainly isolated from the normal oral and pharyngeal flora in man, together with the potentially highly pathogenic species S. pneumoniae that may also be found resident in the upper respiratory tract of healthy humans. The “hyovaginalis group” includes the species isolated from the vaginal and respiratory tracts of domestic animals and birds. Finally, the “bovis group” includes some of the intestinal species showed at Table 2.
1.2.1 Biochemical characteristics of the genus Streptococcus
Genus description from Whiley and Hardie (2009): Streptococci produces non-spore forming, non-motile Gram-positive spherical or ovoid cells, occurring in pairs or in chains when grown in liquid media. Virtually all species are non-pigmented (some strains of S. agalactiae are yellow, orange or brick-red pigmented). Catalase-negative and facultative anaerobic, some species require additional CO2 for growth. The optimal growth temperature is usually about 37°C, with the maximum and minimum varying somewhat among species.
Streptococci are chemoorganotrophic, generally with complex nutrients requirements. The metabolism is Homofermentative with lactic acid as the predominant end product of glucose fermentation, without gas. Mainly unable to produce pyrrolidonyl arylamidase (PYR) and able to produce leucine arylamidase (LAP) there are only occasional exceptions.
13.1.3 Differentiation of enterococci from fecal streptococci
The characteristics most used to differentiate Enterococcus from intestinal Streptococcus are shown in Table 3.
The differentiation of Enterococcus from other related genera is achieved via the species identification. How-ever, for practical purposes the following approach can be used (Svec and Devriese, 2009): catalase negative, Gram positive cocci, showing good growth on Enterococcus selective media containing 0.4% of sodium azide, and able to growth in 6.5% (w/v) of NaCl, can be identified presumptively as belonging to the genus Enterococcus. Typically, only strains of the S. bovis group show colony characteristics similar of those of the classical “enterococci” on these selective media, but these strains do not growth in 6.5% of NaCl. However, it should be kept in mind that this procedure excludes several enterococcal species.
1.4 Methods of analysis
According to the 4th Edition of the Compendium of Methods for the Microbiological Examination of Foods (Hartman et al., 2001) the method most commonly used in the analysis of foods is surface plating on KF Streptococcus Agar. KF Agar is a differential selective medium that uses sodium azide as main selective agent, triphenyltetrazolium chloride (TTC) as differential agent and the carbohydrates maltose (2%) and lactose (0.1%) as carbon sources. In this growth medium, E. faecalis reduces TTC to its formazan derivative, imparting a deep red color to the colonies. Other enterococci may also grow, but are poor reducers of TTC, producing pink colonies. The reaction of the new Enterococcus species, discovered only after the genus was created, has not been described so far.
The majority of other lactic acid bacteria are partially or totally inhibited on KF Agar, although some strains of Pediococcus, Lactobacillus and Aerococcus may grow and produce slightly pink colonies. Sodium azide may also inhibit several strains of S. equinus and, possibly, several of the newly discovered species of the Enterococcus genus (Hartman et al., 2001).
For dairy products, 17th Edition of the Standard Methods for the Examination of Dairy Products (Frank & Yousef, 2004) recommends pour plate using Citrate Azide Agar as culture medium, with an overlay of the same medium and incubation at 37°C/48–72 h. The enterococci colonies appear blue, stained by the tetrazolium blue dye contained in the medium.
Counts may also be obtained by the Most Probable Number (MPN) method, although this method is less
Table.3 Characteristics most used to differentiate Enterococcus from intestinal Streptococcus (Svec and Devriese, 2009, Whiley and Hardie, 2009).

Symbols for Streptococcus species results (except S. henryi and S. caballi): +, <85% positive; d, different strains give different reactions (16–84% positive); –, 0–15% positive; w, weak reaction; ND, not determined (Whiley and Hardie, 2009).
a Data from Milinovich et al. 2008.
b PYR = pyrrolidonyl arylamidase = pyrrolidonyl aminopeptidase production; LAP = leucine arylamidase = leucine aminopeptidase production.
c A positive result is traditionally considered to be typical for the genus, but this does not apply to some of the more recently described species (Svec and Devriese, 2009).
frequently used in the examination of foods. The culture medium is KF Streptococcus in the form of broth and growth accompanied by the color change to yellow with-out the formation of foam (gas) is considered confirmative (Hartman et al., 2001, Zimbro & Power, 2003).
For water analysis the counts of intestinal streptococci and enterococci may be obtained by the membrane filtration method, executed as described in the Section 9230 of the 21st Edition of the Standard Methods for the Examination of Water and Wastewater (Hunt & Rice, 2005). Its main application is the analysis of mineral or natural water, intended for human consumption, with filtration of 100 ml of the sample. The culture medium allowing the simplest procedure is m-Enterococcus Agar, a differential selective medium that uses sodium azide as selective agent for Gram-negative bacteria, triphenyltetrazolium chloride (TTC) as differential agent and glucose as the carbohydrate for fermentation. Typical colonies are confirmed by the catalase test and cell morphology (Gram stain). Intestinal streptococci and enterococci are not differentiated.
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
Lancefield, R.C. (1933) A serological differentiation of human and other groups of hemolytic streptococci. Journal of Experimental Medicine, 57, 591–595.
Leclerc, H, Devriese, L.A. & Mossel, D.A.A. (1996) Taxonomical changes in intestinal (faecal) enterococci and streptococci: con-sequences on their use as indicators of faecal contamination in drinking water. Journal of Applied Bacteriology, 81, 459–466.
Svec, P. & Devriese, L.A. (2009) Genus I Enterococcus (ex Thiercelin and Jouhaud 1903) Schleifer and Kilpper-Bälz 1984. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Sys-tematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 594–607.
Collins, M.D., Jones, D., Farrow, J.A.E., Kilpper-Bälz, R. & Sch-leifer K.H. (1984) Enterococcus avium nom. rev., comb. nov.; E. casseliflavus nom. rev., comb. nov.; E. durans nom. rev., comb. nov.; E. gallinarum comb. nov.; and E. malodoratus sp. nov. Inter-national Journal of Systematic Bacteriology, 34, 220–223.
DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkul-turen) (2011) Bacterial Nomenclature Up-to-Date. Available from: http://old.dsmz.de/microorganisms/bacterial_nomenclature. php? [Acessed 16th January 2012].
Euzéby, J.P. (2011) List of Prokaryotic names with Standing in Nomenclature. [Online] Available from: http://www.bacterio.cict. fr/ [Accessed 14th December 2011].
Whiley, R.A. & Hardie, J.M. (2009) Genus I Streptococcus Rosen-bach 1884. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 655–711.
Milinovich, G.J., Burrell, P.C., Pollitt, C.C., Bouvet, A. & Trott, D.J. (2008) Streptococcus henryi sp. nov. and Streptococcus caballi sp. nov., isolated from the hindgut of horses with oligofructose-induced laminitis. International Journal of Systematic and Evolutionary Microbiology, 58, 262–266.
Tanasupawat, S., Sukontasing, S. & Lee, J.S. (2008) Enterococcus thailandicus sp. nov., isolated from fermented sausage (‘mum’) in Thailand. International Journal of Systematic and Evolutionary Microbiology, 58, 1630–1634.
Rahkila, R., Johansson, P., Säde, E. & Björkroth, J. (2011) Identification of enterococci from broiler products and a broiler processing plant and description of Enterococcus viikkiensis sp. nov. Applied and Environmental Microbiology, 77, 1196–1203.
Euzéby (2009) Enterococcus. In: Euzéby, J.P. Dictionnaire de Bactéri-ologie Vétérinaire. [Online] France Available from: http://www. bacterio.cict.fr/bacdico/ee/enterococcus.html [Accessed 14th December 2011].
Craven, H.M., Eyles, M.J. & Davey, J.A. (1997) Enteric indicator organisms in foods. In: Hocking, A.D., Arnold, G., Jenson, I., Newton, K. & Sutherland, P. (eds). Foodborne Microrganisms of Public Health Significance. Sydney, Australia, Australian Institute of Food Science and Technology Inc. Chapter 5, pp. 139–168.



|
|
|
|
لشعر لامع وكثيف وصحي.. وصفة تكشف "سرا آسيويا" قديما
|
|
|
|
|
|
|
كيفية الحفاظ على فرامل السيارة لضمان الأمان المثالي
|
|
|
|
|
|
|
شعبة مدارس الكفيل: مخيَّم بنات العقيدة يعزِّز القيم الدينية وينمِّي مهارات اتخاذ القرار لدى المتطوِّعات
|
|
|