

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Presence/absence method for Salmonella in foods
المؤلف:
SILVA, N.D .; TANIWAKI, M.H. ; JUNQUEIRA, V.C.A.; SILVEIRA, N.F.A. , NASCIMENTO , M.D.D. and GOMES ,R.A.R
المصدر:
MICROBIOLOGICAL EXAMINATION METHODS OF FOOD AND WATE A Laboratory Manual
الجزء والصفحة:
10-3-2016
5718
Presence/absence method for Salmonella in foods
The International Organization for Standardization method is applicable to products intended for human consumption or for the feeding of animals, and to environmental samples in the area of food production and food handling. It may not recover all Salmonella Typhi and Paratyphi.
1 . Material required for analysis
• Buffered Peptone Water (BPW)
• Rappaport-Vassiliadis Soya (RVS) Broth
• Muller Kauffmann Tetrathionate Novobiocin Broth (MKTTn)
• Xylose Lysine Desoxycholate Agar (XLD) plates
• 2nd Salmonella selective isolation medium plates
(chosen by the laboratory)
• Nutrient Agar (NA) plates
• Nutrient Agar (NA) semi-solid tubes
• Triple Sugar Iron Agar (TSI) slants
• Christensen Urea Agar slants
• Decarboxylation Medium 0.5% L-Lysine
• Tryptone (Tryptophane) Broth
• MR-VP Broth
• β-Galactosidase Reagent (ONPG Reagent) (o-Nitrophenyl-β-D-galactopyranoside)
• Indole Kovacs Reagent
• Voges Proskauer (VP) Reagents ISO (1-naphthol solution, 40% KOH solution, creatine solution)
• Salmonella polyvalent somatic (O) antisera
• Salmonella polyvalent flagellar (H) antisera
• Salmonella Vi antiserum
• Laboratory incubator set to 37 ± 1°C
• Water bath set to 37 ± 1°C
• Laboratory incubator or water bath set to 41.5 ± 1°C
2 . Procedure
A general flowchart for detection of Salmonella in foods using the presence/absence method ISO 6579:2002/Cor.1:2004/Amd.1:2007 is shown in Figure 1.
a) Pre-enrichment: homogenize 25 g or 25 ml of sample with 225 ml of Buffered Peptone Water (BPW). Incubate at 37 ± 1°C/18 ± 2 h.
Note a.1) For pre-enrichment of cocoa and products containing more than 20% cocoa, use BPW supplemented with casein (50 g/l) (avoid the use of acid casein) or skim milk powder (100 g/l). If the foodstuff is likely to be highly contaminated with Gram positive flora, incubate BPW for 2 h, add 0.018 g/l of brilliant green and continue incubation.
Note a.2) For pre-enrichment of acidic and acidifying food-stuffs, use double-strength BPW, to ensure pH above 4.5 during incubation.
b) Selective enrichment: After incubation transfer 0.1 ml of BPW into 10 ml of Rappaport-Vassiliadis Soya Broth (RVS) and 1 ml into 10 ml of Muller Kauffmann Tetrathionate Novobiocin Broth (MKTTn). Incubate the RVS tubes at 41.5 ± 1°C/24 ± 3 h and the MKTTn tubes at 37 ± 1°C/24 ± 3 h.
c) Selective-differential plating: From each RVS and MKTTn culture, streak a loopful on Xylose Lysine Desoxycholate Agar (XLD). Proceed in the same manner with a second Salmonella selective isolation medium chosen by the laboratory. Incubate XLD plates (inverted) at 37 ± 1°C/24 ± 3 h. Incubate the second isolation medium plates according to manufacturers’ instructions.
Note c.1) ISO 6579 recommends a second isolation medium complementary to XLD and especially appropriate for the isolation of lactose positive Salmonella, Salmonella Typhi and Salmonella Paratyphi strains. Examples of isolation media to be used are Bismuth Sulfite Agar (BS) and Brilliant Green Agar (BG).
Note c.2) ISO 6579 recommends a large-size Petri dish (diameter 140 mm) for isolation of Salmonella. In the absence of large dishes, the use of two small dishes (90–100 mm) is recommended, one after the other, without flaming the loop.
d) Colony selection and purification: After the incubation period, examine the plates for typical Salmonella colonies on XLD, which are pink, with or without black centers. Many Salmonella cultures may produce colonies with large black centers or may appear as almost completely black colonies. Atypically, a few lactose positive Salmonella cultures produce yellow colonies with or without black centers.
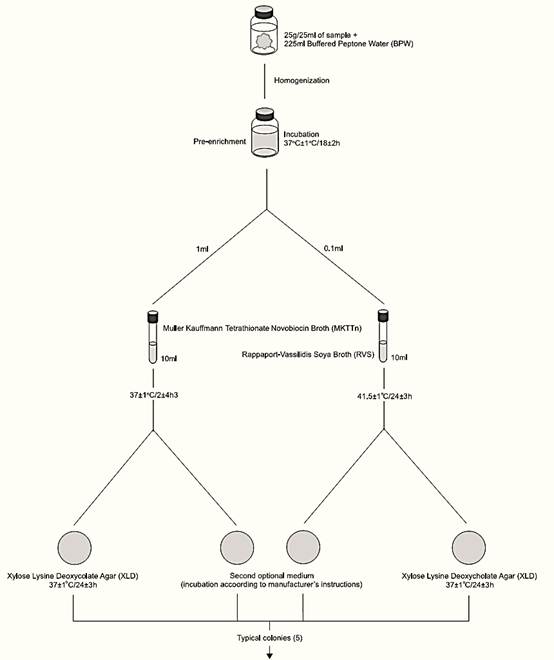
Figure: 1 continued
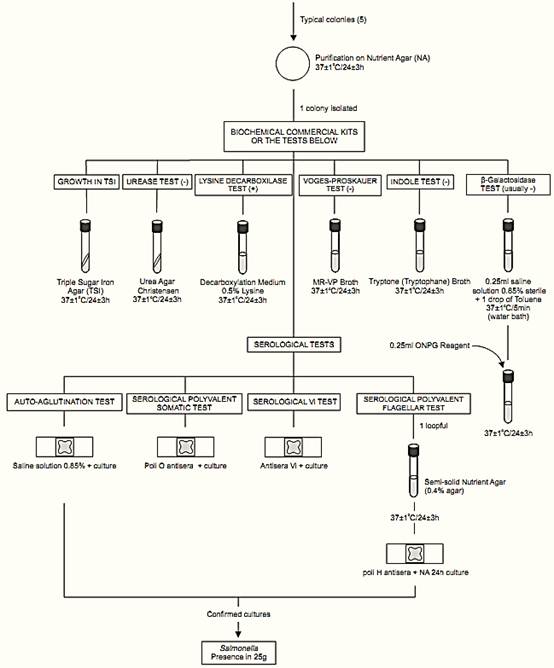
Figure.1 Scheme of analysis for detection of Salmonella in foods using the presence/absence method ISO 6579:2002/Cor.1:2004/Amd.1:2007
may produce colonies with large black centers or may appear as almost completely black colonies. Atypically, a few lactose positive Salmonella cultures produce yellow colonies with or without black centers.
Follow manufacturers’ instructions to select typical colonies on the second isolation medium. Select five typical colonies of Salmonella from each selective agar. In the absence of typical Salmonella colonies, select atypical. Submit one pure culture to biochemical and serological confirmation. If the first is negative, submit the further four. It is recommended that at least five typical or suspected colonies be identified in the case of epidemiological studies.
For purification, streak the selected colonies onto the surface of Nutrient Agar (NA) plates. Incubate the plates at 37 ± 1°C/24 ± 3 h.
e) Biochemical confirmation: Use pure cultures from the NA plates for biochemical and serological tests below. As an alternative to conventional biochemical tests, commercial biochemical kits can be used. Commercial biochemical kits should not be used as a substitute for serological tests.
e.1) TSI reactions: Inoculate each suspect culture into Triple Sugar Iron Agar (TSI) tubes by streaking the slant and stabbing the butt. Incubate at 37 ± 1°C/24 ± 3 h. Cap the tubes loosely to maintain aerobic conditions (to prevent excessive H2S production). After the incubation period, examine the tubes for typical Salmonella reactions:
alkaline (red) slant and acid (yellow) butt with gas formation (bubbles) and (in about 90% of the cases) H2S formation (agar blackening). When lactose-positive Salmonella strains are isolated, the TSI slant is yellow. Thus, preliminary confirmation should not be based on the result of TSI reactions only.
e.2) Urease test: Inoculate Urea Agar Christensen by streaking the slant. Incubate at 37 ± 1°C/24 ± 3 h and examine periodically. Positive reaction is indicated by change in the color of medium to rose-pink. Negative reaction is indicated by lack of color change. The reaction is often apparent after two to four hours. Most Salmonella strains are urease negative. Include an uninoculated tube as control because uninoculated tubes of Christensen Urea Agar may turn rose-pink on standing.
e.3) L-Lysine decarboxylase test: Inoculate the cultures into tubes of Decarboxylation Medium 0.5% L-Lysine and incubate the tubes at 37 ± 1°C/24 ± 3 h. Positive reaction is indicated by turbidity and purple color throughout the medium. Negative reaction is indicated by yellow color throughout the medium. Most Salmonella strains are lysine decarboxylase positive, but Paratyphi serotype strains are negative.
e.4) Voges-Proskauer (VP) test: Inoculate each culture into 3 ml tubes of MR-VP Broth and incubate the tubes at 37 ± 1°C/24 ± 3 h. After incubation add the Voges Proskauer (VP) Reagents ISO as follows: two drops of creatine solution, three drops of 1-naphthol solution and two drops of 40% KOH solution. Shake after addition of each reagent.
Development of a pink to red color within 15 min is indicative of a positive result. Lack of pink to red color is indicative of a negative result. Salmonella strains are VP negative.
e.5) Indole test: Inoculate the cultures into 5 ml tubes of Tryptone (Tryptophane) Broth. Incubate the tubes at 37 ± 1°C/24 ± 3 h. After incubation add 1 ml Indole Kovacs Reagent. The formation of a red ring at the broth surface is indicative of a positive result. A yellow-brown ring is indicative of a negative result. Most Salmonella strains are indole negative.
e.6) β-galactosidase detection: Suspend a loopful of the culture in a tube containing 0.25 ml of saline solution. Add a drop of toluene, shake the tube and incubate at 37 ± 1°C/5 min (water bath). Add 0.25 ml of the β-Galactosidase Reagent (ONPG Reagent) (o-Nitrophenyl-β-D-galactopyranoside) and mix. Incubate at 37 ± 1°C/24 ± 3 h (water bath) and examine periodically. Development of a yellow color (often within 20 min) is indicative of a positive result. Lack of a yellow color is indicative of a negative result. Most Salmonella enterica subsp. enterica strains are negative
Note e.6.1) As an alternative to the conventional test, commercial paper discs can be used, following manufacturers’ instructions: Taxo™ ONPG Discs (BBL 231249), ONPG Discs (Oxoid DD013), ONPG Discs (Fluka 49940).
f ) Serological confirmation
f.1) Elimination of autoagglutinable strains: Emulsify a loopful of the culture to be tested in a drop of saline solution on a glass slide. Tilt the mixture in back-and-forth motion for 30–60s and observe against a dark background. If agglutination occurs the strain is considered autoagglutinable and should not be submitted to serological tests.
f.2) Serological polyvalent somatic (O) test: Follow the same procedure described for elimination of autoagglutinable strains (f.1), using one drop of Salmonella polyvalent somatic (O) antisera instead of the saline solution. If agglutination occurs, the reaction is considered positive (for pre-tested non-au-to-agglutinable strains).
Note f.2.1) The polyvalent somatic antisera should contain, at least, antibodies for the anti-genic factors of the serological somatic groups A, B, C1, C2, D, E1 and E4. Preferably, they should also contain antibodies for the Vi capsular antigen, which, if present, will mask the somatic antigens. When selecting and purchasing commercial brands, choose suppliers that pro-vide on the label or product information sheet a detailed list of the factors that are detected by the pool of antibodies present. For example: the Becton Dickinson (BD Difco 222641) Serum indicates the presence of antibodies for the Vi antigen and for the antigenic factors of somatic serogroups A to I (O:1 a O:16, O:19, O:22 a O:25 and O:34).
f.3) Serological Vi test: Follow the same procedure described for elimination of autoagglutinable strains (f.1), using one drop of the Vi antiserum instead of the saline solution. If agglutination occurs, the reaction is considered positive (for pre-tested non-autoagglutinable strains).
Note f.3.1) Some commercial brands of polyvalent somatic antisera also contain antibodies for the Vi capsular antigen. In this case is not necessary to test separately.
f.4) Serological polyvalent flagellar (H) test: Transfer the culture to a semi-solid Nutrient Agar (NA) tube. Incubate at 37 ± 1°C/24 ± 3 h and test for H antigens. Follow the same procedure described for elimination of autoagglutinable strains (f.1) using one drop of Salmonella polyvalent flagellar (H) antisera instead of the saline solution. If agglutination occurs, the reaction is considered positive (for pre-tested non-autoagglutinable strains).
Note f.4.1) Some commercial brands of antisera contain antibodies for the polyvalent somatic and the polyvalent flagelar antigens, but the ISO polyvalent flagellar test should be performed separately, because the culture is cultivated in semi soli medium before the test (flagella enrichment).
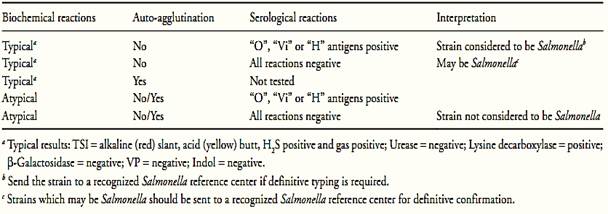
g) Interpretation of results: Follow orientation shown in Table 1.
Table 1 Guide for the interpretation of Salmonella confirmatory tests according the method ISO 6579:2002/Cor.1:2004/Amd.1:2007.
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
International Organization for Standardization (2007) ISO 6579:2002/Cor 1:2004/Amd 1:2007. Microbiology of food and animal feeding stuffs – Horizontal method for the detection of Sal-monella spp. 4th edition: 2002, Corrigendum 1:2004, Amend-ment 1:2007. Geneva, ISO.
 الاكثر قراءة في البكتيريا
الاكثر قراءة في البكتيريا
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















