

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Bacillus cereus
المؤلف:
SILVA, N.D .; TANIWAKI, M.H. ; JUNQUEIRA, V.C.A.; SILVEIRA, N.F.A. , NASCIMENTO , M.D.D. and GOMES ,R.A.R
المصدر:
MICROBIOLOGICAL EXAMINATION METHODS OF FOOD AND WATE A Laboratory Manual
الجزء والصفحة:
9-3-2016
13857
Bacillus cereus
1- Introduction
Bacillus cereus is a pathogenic bacterium, which causes foodborne diseases classified by the International Commission on Microbiological Specifications for Foods (ICMSF, 2002) in Risk Group III: “diseases of moderate hazard usually not life threatening, normally of short duration without substantial sequelae, causing symptoms that are self-limiting but can cause severe discomfort”.
1.1 B. cereus Group
Bacillus cereus, Bacillus anthracis, Bacillus thuringiensis, Bacillus mycoides, Bacillus pseudomycoides and Bacillus weihenstephanensis constitute a group of Bacillus species (B. cereus Group) very closely related and, from a practical point of view, difficult to distinguish from each other. They are Gram-positive rods, spore-forming, facultative anaerobic and each species is differentiated from B. cereus by, basically, for one single characteristic (Bennett and Belay, 2001, Euzéby, 2003). The main characteristics of B. cereus Group are summarized in Table 1.
B. anthracis is pathogenic to man as well as B. cereus. According to Euzéby (1998) B. anthracis is a feared bacterium due to its high potential for use as a biological weapon. It causes a disease that is known as anthrax, which affects herbivorous animals (particularly ruminants) and which may be transmitted to man, mainly through contact with infected animals. Transmission occurs through exposure to respiratory, cutaneous or gastrointestinal secretions, and poses an important risk to animal health professionals, breeders and shearers. Differentiation from B. cereus (Tallent et al., 2012): B. anthracis is nonmotile and usually non-hemolytic after 24 h of incubation. A few B. cereus strains are also nonmotile but the cultures usually are strongly hemolytic and produce a 2–4 mm zone of complete (β) hemolysis surrounding growth after 24 h.
B. thuringiensis is pathogenic to insects, and as such has been used as a biological control agent (bio-insecticide) in agriculture for more than 30 years (Valadares-Inglis et al., 1998). During the spore-forming process, the cells produce crystalline inclusions (parasporal crystals), composed of one or more polypeptides (delta-endotoxins), which are encoded by Cry genes. The toxins are active against the larvae of the insects of the Lepidoptera, Coleoptera and Diptera orders, as well as against nematodes, mites and ticks (Dean, 1984). They do not affect man, animals or plants (Souza et al., 1999).
B. cereus does not produce protein toxin crystals. B. mycoides exhibits a typical colony morphology on solid culture media (rhizoid colonies), reminding fungal growth. From the point of inoculation onwards, multiplication occurs in chains of cells linked end to end, forming long radial filaments bending to the right or to the left (Di Franco et al., 2002). The colonies have the appearance of roots, from which derives the term rhizoid.
B. pseudomycoides is a new species, proposed by Nakamura (1998), formed by a group of strains of B. mycoides with a different fatty acids composition. The morphological, physiological and growth characteristics are indistinguishable from those of B. mycoides, including the rhizoid growth on solid culture media.
B. weihenstephanensis is a new species, proposed by Lechner et al. (1998). It is formed by psychrotrophic strains of B. cereus, separated from the mesophilic strain, which continue belonging to the cereus species. It exhibits all typical characteristics of B. cereus, from which it differs only by its capacity to grow between 4 and 7ºC and its inability to grow at 43ºC. According to Euzéby (2003) no intoxications have been formally attributed to B. weihenstephanensis, but it is likely that the pathogenicity of this species is comparable to that of B. cereus.
Table 1 Differential characteristics of the species of Bacillus cereus group (Tallent et al., 2012)a.
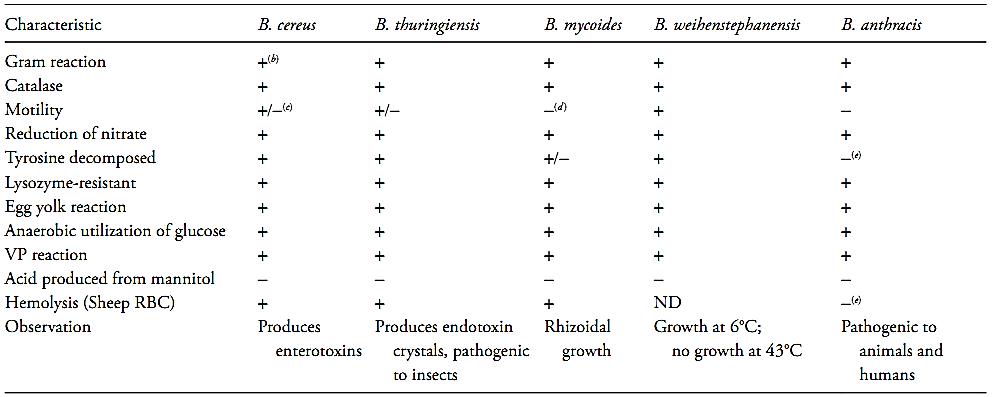
a The data were taken from BAM Online (Tallent et al., 2012) which does not deal with B. mycoides and B. pseudomycoides separately.
b +, 90–100% of strains are positive.
c +/−, 50–50% of strains are positive.
d −, 90–100% of strains are negative.
e −, Most strains are negative. ND, not determined
1.2 Main characteristics of B. cereus
The strains of B. cereus are Gram positive, usually motile rods, occurring singly, in pairs and long chains. They form ellipsoidal, sometimes cylindrical, subterminal, sometimes paracentral, spores which do not swell the sporangia. Catalase-positive, oxidase-negative, nitrate is reduced by most strains. Egg yolk reaction is positive and tyrosine is decomposed. Resistant to 0.001% lysozyme. Facultative anaerobic, acid without gas is produced from glucose and a limited range of other carbohydrates. B. weihenstephanensis is phenotypically similar (Logan and De Vos (2009).
According to ICMSF (1996) which does not separate B. weihenstephanensis from B. cereus, the optimal growth temperature is between 30 and 40ºC, with a minimum of 4ºC and a maximum of 55ºC. The optimal pH value lies between 6.0 and 7.0, with a minimum of 5.0 and a maximum of 8.8. The minimum water activity is 0.93. According to Logan and De Vos (2009), who separate B. weihenstephanensis from B. cereus, the minimum temperature for B.cereus growth is usually 10–20°C, but psychrotolerant strains growing at 6°C have been isolated. The maximum growth temperature is 40–45°C, with the optimum at 37°C. B. weihenstephanensis characteristically grows at 7°C and does not grow at 43°C.
The spores of B. cereus have a level of heat resistance comparable to that of other spores of mesophilic bacteria, with a D121ºC value between 0.03 and 2.35 min and a z value between 7.9 and 9.9ºC (in 0.067 M phosphate buffer). In rice broth at 100ºC, they resist for 4.2 to 6.3 min (ICMSF, 1996).
The diseases caused by B. cereus are intoxications, which result from the ingestion of toxins formed in the food as a result of the multiplication of cells. Two types of diseases are known:
One is the diarrheic syndrome, characterized by abdominal pain and diarrhea, with an incubation period from eight to 16 hours and onset of symptoms 12 to 24 hours after exposure. This disease is caused by the diarrheic toxin, a heat-sensitive protein, inactivated by heating at 56°C/5 min (Bennett and Belay, 2001).
The other disease known to be caused by B. cereus is the emetic syndrome, characterized by nausea and vomiting, beginning between one and five hours after the contaminated food is consumed. Diarrhea is not the predominant symptom in this case, although it may occur. It is caused by the emetic toxin, a heat-resistant peptide which resists cooking and, also, much more severe heat treatments, such as 120°C for more than one hour (Bennett and Belay, 2001). The optimal temperature for the production of the emetic toxin in rice is 25–30ºC (ICMSF, 1996).
According to Bennett and Belay (2001) the presence of B. cereus in foods does not represent a health hazard, unless it is allowed to multiply and reach populations greater than 105 viable cells per gram. The foods most frequently implicated in outbreaks are either cooked products or products containing cooked ingredients, particularly those rich in starch or proteins, such as cooked rice, cooked pasta, cooked vegetables, soups, vegetable salads, seed sprouts, puddings and cooked meats. Cooking activates the spores and, if refrigeration is not appropriate, these spores may germinate and produce toxins.
1.3 Methods of analysis
B. cereus counts in foods can be done by the direct plate count method, which is the most commonly used, or by the most probable number method, the latter being recommended for cases in which counts lower than 103 CFU/g are expected.
In direct plating, the most frequently used medium is Mannitol Egg Yolk Polymyxin Agar (MYP), which combines polymyxin as selective agent and egg yolk and mannitol as differential agents. The production of colonies with a strong reaction of egg yolk (lecithinase activity), characterized by a large precipitation halo, is typical for bacilli of the B. cereus Group. The non-fermentation of mannitol gives the halo surrounding the colony a milky pink color.
Another recommended medium is Kim-Goepfert (KG) Agar, which has the same level of sensitivity and selectivity as MYP, but is much less used. The colonies that grow on the KG medium are identical to those growing on MYP, but do not show the typical color, since the medium does not contain mannitol. Formulated to stimulate the formation of free spores after 20–24 h incubation, it allows for immediate confirmation of the identity of the colonies (directly from the incubated plates), by staining both the spores and intracellular lipid globules (rapid confirmatory test developed by Holbrook & Anderson). To apply this test to the colonies obtained on MYP, it is necessary to subculture the culture on Nutrient Agar. This way, KG is a faster alternative for the enumeration of B. cereus.
Another advantage of KG is that other Bacillus species that produce lecithinase, such as B. polymyxa, are unable to form lecithinase on this nutritionally poor medium. Confirmation of typical colonies includes two groups of testes, the first to verify whether the isolated culture belongs to the B. cereus Group and the second to differentiate B. cereus from the other bacilli of the Group.
To confirm the culture as pertaining to the B. cereus Group, the 4th Edition of the Compendium of Methods for the Microbiological Examination of Foods (Bennett & Belay, 2001) recommends the rapid confirmatory test of Holbrook & Anderson (1980), a technique that com-bines the Ashby’s spore stain and the Burdon’s intracellular fat stain. In the Holbrook & Anderson method, isolation of B. cereus is achieved in PEMBA (Polymyxin Pyruvate Egg-Yolk Bromothymol Blue Agar). According to the authors, only B. cereus, among the Bacillus species capable of growth on PEMBA, present intracellular lipid globules. Due to the similarity in composition and differential characteristics, PEMBA may be substituted by KG, in the method described by the Compendium.
Colonies presenting typical characteristics on MYP or KG can also be confirmed as pertaining to the B. cereus Group by biochemical assays. The most typical characteristics of the group are determined, including the test of anaerobic utilization of glucose, the tyrosin decomposition test, the VP test, the nitrate test and the lysozyme resistance test.
To differentiate B. cereus from the other bacilli of the Group, the tests to be used are those that verify respectively the following conditions: the production of intracellular toxin crystals, rhizoid growth, and hemolytic activity.
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
ICMSF (International Commission on Microbiological Specifications for Foods) (2002) Microorganisms in Foods 7. Microbiological Testing in Food Safety Management. New York, Kluwer Academic/Plenum Publishers.
Euzéby, J.P. (2003). Systématique des espèces placées dans le “groupe Bacillus cereus”. In: Euzéby, J.P. Dictionnaire de Bactériologie Vétérinaire. [Online] France. Available from http://www.bacterio.cict. fr/bacdico/bb/cereusgroupe.html [Accessed 24th October 2011].
Tallent, S.M., Rhodehamel, E.J., Harmon, S.M. & Bennett, R.W. (2012) Bacillus cereus. In: FDA (ed.) Bacteriological Analytical Manual, Chapter 14. [Online] Silver Spring, Food and Drug Administration. Available from: http://www.fda.gov/Food/Sci-enceResearch/LaboratoryMethods/BacteriologicalAnalytical-Manual BAM/default.htm [accessed 10th April 2012].
Valadares-Inglis, M.C., Souza, M.T. & Shiler, W. (1998) Engenharia genética de microrganismos agentes de controle biológico. In: Melo, I.S. & Azevedo, J.L. (eds). Controle Biológico. Volume 1. Jaguariúna, Embrapa-CNPMA, pp. 208–217.
Dean, D.H. (1984) Biochemical genetics of the bacterial insect-control agent Bacillus thuringiensis: basic principles and prospect for genetic engineering. Biotechnology and Genetic Engineering Reviews, 2, 341–363.
Di Franco, C., Beccari, E., Santini, T. Pisaneschi, G. & Tecce, G. (2002) Colony shape as a genetic trait in the pattern-forming Bacillus mycoides. BMC Microbiology, 2 (1), 33–48.
Lechner, S., Mayr, R, Francis, K.P., Prub, B.M., Kaplan, T., Stewart, G.S.A.B & Scherer, S. (1998) Bacillus weihenstephanensis sp. nov. is a new psychrotolerant species of the Bacillus cereus group. Inter-national Journal of Systematic Bacteriology, 48, 1373–1382.
 الاكثر قراءة في البكتيريا
الاكثر قراءة في البكتيريا
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















