
The valence bond (VB) model of bonding in H2
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
Organic Chemistry
المصدر:
Organic Chemistry
 الجزء والصفحة:
p27
الجزء والصفحة:
p27
 8-3-2016
8-3-2016
 1779
1779
The valence bond (VB) model of bonding in H2
Valence bond theory considers the interactions between separate atoms as they are brought together to form molecules. We begin by considering the formation of H2 from two H atoms, the nuclei of which are labelled HA and HB, and the electrons of which are 1 and 2, respectively.
When the atoms are so far apart that there is no interaction between them, electron 1 is exclusively associated with HA, while electron 2 resides with nucleus HB. Let this state be described by a wavefunction ψ1.
When the H atoms are close together, we cannot tell which electron is associated with which nucleus since, although we gave them labels, the two nuclei are actually indistinguishable, as are the two electrons. Thus, electron 2 could be with HA and electron 1 with HB. Let this be described by the wavefunction ψ2.
Equation 1.1 gives an overall description of the covalently bonded H2 molecule; ψcovalent is a linear combination of wavefunctions ψ1 and ψ2. The equation contains a normalization factor, N (see Box 1.4). In the general case where:

 (1.1)
(1.1)
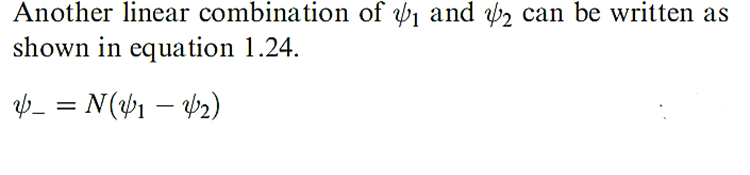
 (1.2)
(1.2)
In terms of the spins of electrons 1 and 2 ,ψ+ corresponds to spin-pairing, and ψ- corresponds to parallel spins (nonspin- paired). Calculations of the energies associated with these states as a function of the inter nuclear separation of HA and HB show that, while  - represents a repulsive state (high energy), the energy curve for ψ + reaches a minimum value when the inter nuclear separation, d, is 87pm and this corresponds to an H-H bond dissociation energy, ΔU, of 303 kJ mol -1. While these are near enough to the experimental values of d = 74pm and ΔU = 458 kJ mol-1 to suggest that the model has some validity, they are far enough away from them to indicate that the expression for ψ+ needs refining.
- represents a repulsive state (high energy), the energy curve for ψ + reaches a minimum value when the inter nuclear separation, d, is 87pm and this corresponds to an H-H bond dissociation energy, ΔU, of 303 kJ mol -1. While these are near enough to the experimental values of d = 74pm and ΔU = 458 kJ mol-1 to suggest that the model has some validity, they are far enough away from them to indicate that the expression for ψ+ needs refining.
The bond dissociation energy (ΔU) and enthalpy (ΔH) values for H2 are defined for the process:
H2(g) → 2H(g)
Improvements to equation 1.1 can be made by:
- allowing for the fact that each electron screens the other from the nuclei to some extent;
- taking into account the possibility that both electrons 1 and 2 may be associated with either HA or HB, i.e. allowing for the transfer of one electron from one nuclear centre to the other to form a pair of ions HA+ HB- or HA-HB+.
The latter modification is dealt with by writing two additional wavefunctions, ψ3 and ψ4 (one for each ionic form), and so equation 1.1 can be rewritten in the form of equation 1.3. The coefficient c indicates the relative contributions made by the two sets of wavefunctions. For a homonuclear diatomic such as H2, the situations described by ψ 1 and  2 are equally probable, as are those described by ψ3
2 are equally probable, as are those described by ψ3
and ψ4.

 (1.3)
(1.3)
Since the wavefunctions ψ1 and ψ2 arise from an internuclear interaction involving the sharing of electrons among nuclei, and  3 and ψ4 arise from electron transfer, we can simplify equation 1.3 to 1.4 in which the overall wavefunction, ψ molecule, is composed of covalent and ionic terms.
3 and ψ4 arise from electron transfer, we can simplify equation 1.3 to 1.4 in which the overall wavefunction, ψ molecule, is composed of covalent and ionic terms.

 (1.4)
(1.4)
Based on this model of H2, calculations with c ≈ 0.25 give values of 75pm for d(H–H) and 398 kJ mol-1 for the bond dissociation energy.
Now consider the physical significance of equations 1.3 and 1.4. The wavefunctions ψ1 and ψ2 represent the structures shown in 1.7 and 1.8, while ψ3 and ψ 4 represent the ionic forms 1.9 and 1.10. The notation HA(1) stands for ‘nucleus HA with electron (1)’, and so on.


Dihydrogen is described as a resonance hybrid of these contributing resonance or canonical structures. In the case of H2 , an example of a homonuclear diatomic molecule which is necessarily symmetrical, we simplify the picture to 1.11. Each of structures 1.11a, 1.11b and 1.11c is a resonance structure and the double-headed arrows indicate the resonance between them. The contributions made by 1.11b and 1.11c are equal. The term ‘resonance hybrid’ is somewhat unfortunate but is too firmly established to be eradicated.

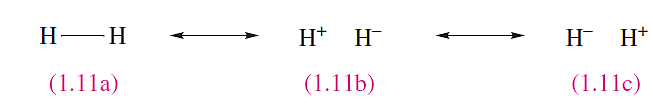
A crucial point about resonance structures is that they do not exist as separate species. Rather, they indicate extreme bonding pictures, the combination of which gives a description of the molecule overall. In the case of H2, the contribution made by resonance structure 1.11a is significantly greater than that of 1.11b or 1.11c. Notice that 1.11a describes the bonding in H2 in terms of a localized two-centre two-electron, 2c-2e, covalent bond. A particular resonance structure will always indicate a localized bonding picture, although the combination of several resonance structures may result in the description of the bonding in the species as a whole as being delocalized .
 الاكثر قراءة في نظريات التآصر الكيميائي
الاكثر قراءة في نظريات التآصر الكيميائي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


