

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Microscopic Approach
المؤلف:
C. Br´ echignac P. Houdy M. Lahmani
المصدر:
Nanomaterials and Nanochemistry
الجزء والصفحة:
p48
28-2-2016
1418
Microscopic Approach
When a surface is created, the atomic planes near the surface are usually displaced. The direction and magnitude of this relaxation depends on the type of material and also the orientation of the surface. In rare gas crystals or ionic crystals, for example, the bonds between the atoms are relatively longrange. In the bulk, the relative position of the atoms is determined by the competition between the mutual repulsion of the nuclei and long-range forces (Coulombic in the case of ionic crystals). In this case, the atomic planes at the surface expand. In the case of face- or body-centered cubic metals, the situation is generally different: for close-packed planes without defects, a very small surface relaxation is generally observed. On the other hand, if a close-packed surface has a certain degree of roughness due to the presence of defects such as steps or islands, or if the surface is not close-packed, the outermost layer will relax considerably towards the core of the material, whilst certain layers further in will move toward the surface. As an example, Fig.1. shows the various motions of the (210) surface planes in platinum. These different relaxations compensate for the fact that the atoms at the surface have fewer near neighbours, whence the electron density is lower there. Relaxation is thus a way of compensating for changes in the electron density in each plane, in such a way as to make it as homogeneous as possible right out to the outermost surface.
Consider now a nanocrystal with approximately spherical shape. Relaxation of the first atomic planes will lead in this case to a pressure exerted by the surface on the core of the nanocrystal, and hence to an increase or a decrease in the lattice parameter. An effect is therefore induced not only by the adsorption state of the surface, but also by the surface itself. This effect is even more marked in semiconductors, where surfaces are generally reconstructed, in order to minimise the number of dangling bonds, which generates large stresses in the first layers.

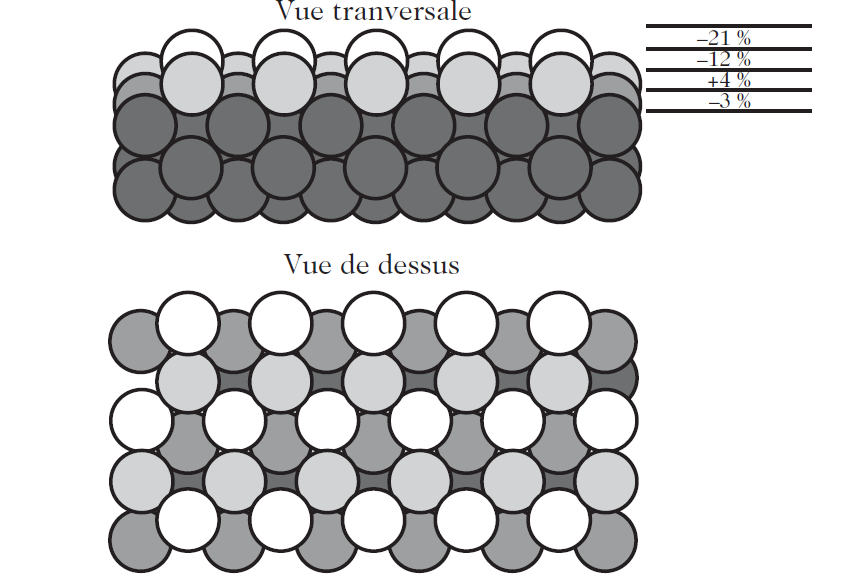
Fig. 1. Schematic description of surface relaxation effects in the case of a Pt (210) surface. These relaxations are deduced from models based on LEED (low energy electron diffraction) studies
In a nanocrystal, these stresses will also contribute to the surface pressure and to the contraction or expansion of atomic bonds. For example, simulations of Ge nanocrystals with surface composed of reconstructed layers have indicated a reduction in the structure parameter of up to 6% for 2-nm nanoparticles. We have limited the discussion here to clean surfaces. In the more complicated case where there is adsorption on nanocrystal surfaces, it is not possible a priori to guess whether one will observe a contraction, or rather an expansion of the surface planes, i.e., a reduction or an increase in the lattice parameter. In the present state of our understanding, only a detailed analysis in each particular case can provide an answer to this question.
 الاكثر قراءة في كيمياء النانو
الاكثر قراءة في كيمياء النانو
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















