


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية | Elementary Thermodynamics of the Grain Size Dependence of Phase Transitions |
|
|
|
Read More
Date: 2-12-2015
Date: 2-12-2015
Date: 3-2-2016
|
Elementary Thermodynamics of the Grain Size Dependence of Phase Transitions
Theory
The various examples described above show that the phase transitions, and more generally the phase diagrams of a material should be considered in a temperature–pressure–grain size space (or rather, reciprocal radius of curvature R for the latter degree of freedom). However, a general theory has not yet been put together. In this context, it is interesting to see what standard thermodynamics has to say about the matter:
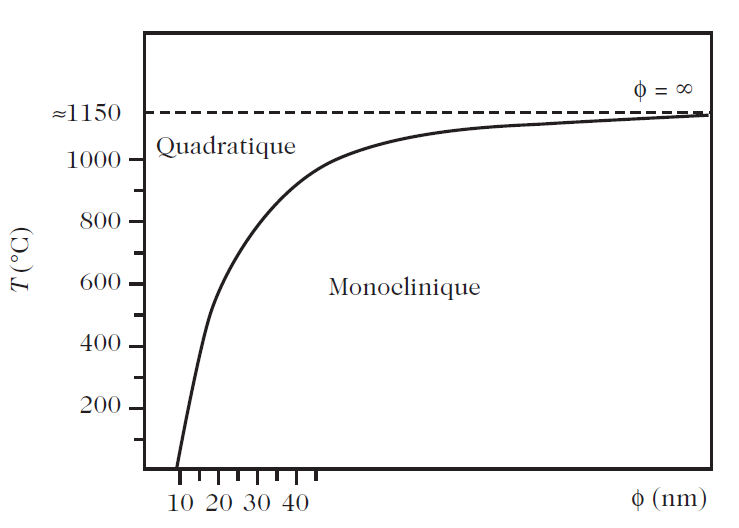
Fig. 2.6. Temperature of the tetragonal–cubic phase transition in BaTiO3 as a function of the grain size. Calculated result from and experimental result from
• The stable states of the system are no longer governed by the free enthalpy G at constant T and P. This role is fulfilled by a generalised free enthalpy function G∗ given by G∗ = G − 2γV/R .
• G∗ is no longer a state function of the system. γ is the surface energy, V the molar volume of the material, and R the radius of the nanocrystal, assumed spherical.
• The equilibrium state of a system made from nanometric grains is no longer obtained by the condition dG = 0, but rather by dG∗ = 0.
• There is therefore equilibrium between the phase α and the phase β of the same body if G∗α = G∗β.
• For given temperature and pressure, there is a critical radius Rc at which the phase transition occurs in nanometric grains.
• So the temperature Tc of the phase transition is, in particular, a function of the radius R of the nanocrystals:
Tc = T(P,R, γα, γβ) . This relation has been clearly demonstrated for the tetragonal–cubic transition in BaTiO3.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|