


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-9-2017
Date: 21-3-2016
Date: 26-5-2020
|
CATIONIC POLYMERIZATION
Monomers with electron-donating groups like isobutylene form stable positive charges and are readily converted to polymers by cationic catalysts. Any strong Lewis acid like boron trifluoride (BF3) or Friedel–Crafts catalysts such as AlCl3 can readily initiate cationic polymerization in the presence of a cocatalyst like water, which serves as a Lewis base or source of protons. During initiation, a proton adds to the monomer to form a carbonium ion, which forms an association with the counterion. This is illustrated for isobutylene and boron trifluoride in Equation below.
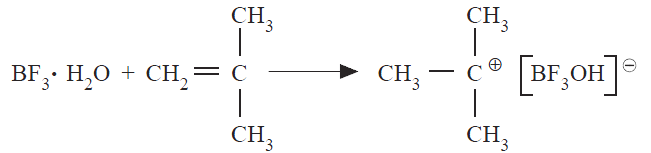
Propagation involves the consecutive additions of monomer molecules to the carbonium ion at the growing chain end. Termination in cationic polymerization usually involves rearrangement to produce a polymer with an unsaturated terminal unit and the original complex or chain transfer to a monomer and possibly to the polymer or solvent molecule. Unlike free-radical polymerization, termination by combination of two cationic polymer growing chains does not occur.
Cationic polymerizations are usually conducted in solutions and frequently at temperatures as low as –80 to –100°C. Polymerization rates at these low temperature conditions are usually fast. The cation and the counterion in cationic polymerization remain in close proximity. If the intimate association between the ion pair is too strong, however, monomer insertion during propagation will be prevented. Therefore the choice of solvent in cationic polymerization has to be made carefully; a linear increase in polymer chain length and an exponential increase in the reaction rate usually occur as the dielectric strength of the solvent increases.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|