


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 22-12-2015
Date: 10-3-2021
Date: 16-6-2021
|
Conotoxins
Perhaps the largest array of channel-binding toxins is produced by marine gastropods of the genus Conus, which includes more than 500 species (1, 2). These animals appear to have developed the ability of generating a very large number of toxin variants starting from a basic peptide structure consisting of relatively few residues (Fig. 1). In such a way, they produce venoms containing complex mixtures of toxins, capable of binding and blocking the activity of several ion channels. Moreover, some conotoxins are capable of binding differentially to the various isoforms of an ion-specific channel, thus providing precious tools to pharmacologists and neuroscientists (1).
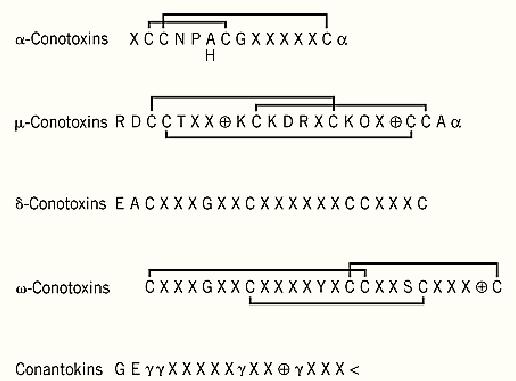
Figure 1. Peptide toxins contained in Conus venoms. Conus spp. produce a highly complex mixture of toxins. Shown here are the consensus sequences and disulfide connections of toxins specific for the acetylcholine receptor (a-conotoxins), sodium channels (m- and d-contoxins), calcium channels (w-conotoxins), and the NMDA glutamate channel (conantokins). O indicates trans-4-hydroxyproline, plus a positively charged residue, g a g-carboxyglutamate, and a an amidated a-carboxyl. The disulfide connectivity of d-conotoxins is not established.
The m-conotoxins are 22 residue-long basic peptides and include three hydroxyproline residues and six cysteines that form three disulfide bonds (Fig. 1). These conotoxins have an ellipsoid shape with the basic residues, which are essential for the binding activity, clustered on one side of the molecule (2, 3) . m-conotoxins bind to the tetrodotoxin binding site of the sodium channel, thereby inhibiting the propagation of the action potential and causing flaccid paralysis. Another family of conotoxins specific for sodium channels is that of the d-conotoxins, whose binding causes an increased conductance of such voltage-gated channels (4).
w-conotoxins act on voltage-gated calcium channels and inhibit calcium entry through the presynaptic membrane, thus preventing the release of neurotransmitters. Their peptide chain consists of 24 to 30 residues and three disulfide bonds (Fig. 1). A frequently used w-conotoxin is GVIA, a 27 residue-long peptide that is very specific for calcium channels containing the a1B subunit, such as the N-type calcium channel present at the neuromuscular junction of vertebrates. The specificity of different w-conotoxins for different vertebrates is exploited in studying the function and anatomical distribution of calcium channels (1).
The largest family of conotoxins is that of the a-conotoxins, 13 to 18 residue-long peptides, whose folding is dominated by two disulfide bonds (Fig. 1). These paralytic neurotoxins bind specifically to the nicotinic acetylcholine receptor and different a-conotoxins are able to distinguish between different neuronal isoforms of the receptor. Conantokins are Conus peptides with yet another channel specificity: They bind to the NMDA-sensitive glutamate channels. They are peptides of 17 to 21 residues with no cysteine but four gamma-carboxyglutamate residues (1) (Fig. 1). Very short Conus peptides are conopressins, which consist of nine amino acid residues with a single disulfide bond and are specific for the vasopressin receptor.
References
1. R. Rappuoli and C. Montecucco (1997) Guidebook to Protein Toxins and Their Use in Cell Biology, Sambrook and Tooze, Oxford University Press, Oxford, UK.
2. B. M. Olivera, G. Miljanich, J. Ramachandran, and M. E. Adams (1994) Ann. Rev. Biochem. 63 823–867.
3. J.-M. Lancelin et al. (1991) Biochemistry 30, 6908–6916.
4. K. Shon (1995) Biochemistry 34, 4913.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
ضمن أسبوع الإرشاد النفسي.. جامعة العميد تُقيم أنشطةً ثقافية وتطويرية لطلبتها
|
|
|