


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 22-4-2021
Date: 17-4-2021
Date: 23-12-2015
|
Chaotropes: Kosmotropes
Compounds that increase and decrease, respectively, the aqueous solubility of proteins are classified as chaotropes and kosmotropes (1). Therefore, the Hofmeister series of ions may be divided into these two categories. For the series of anions, solubilization in water is promoted in the order
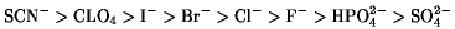
Those on the left-hand side of Cl– generally increase the solubility; they are called chaotropes.
Those on the right-hand side of Cl– act as salting-out agents; they are called kosmotropes. Chaotropes are water-structure breakers; kosmotropes, to the contrary, are water-structure makers. A similar division may be made about the Na+ ion in the cationic Hofmeister series. In the salts, the predominant effect is that of anions. An important characteristic is that the effects are additive for all the species in the solution.
Organic molecules also affect water structure and can be classified as chaotropes and kosmotropes. Thus, urea, glycine, formamide, and acetamide are chaotropes. Organic kosmotropes are sucrose, polyols, the methylacetamines, methyl formamides, and methyl ureas. Except for sugar and the polyols, caution must be exercized in using organic kosmotropes, since their action may reverse itself at high concentration.
The mechanism of action of these molecules in stabilizing or destabilizing protein structure, and as a corollary in acting as salting in or salting out agents, is related to their effects on the orientation of water molecules through polar interactions with the water hydrogen bond donor and acceptor properties. By perturbing or strengthening the interactions between water molecules (maximization of hydrogen bond formation), these molecules exercise their chaotropic or kosmotropic actions. At interfaces, the water structure is already perturbed by the nonavailability at the surface of other water molecules for hydrogen bond formation. This is true for the water–air interface. Similar perturbations may occur at interfaces with proteins and other biological entities—hence, the effect on their properties. Control of reactions by the chaotropic/kosmotropic effect is very widespread in biological systems. Its principal diagnosis is that the compound which affects a particular process must be present at a high concentration (local concentration for cell compartments and organelles).
References
1. K. D. Collins and M. W. Washabaugh (1985) Quart. Rev. Biophys. 18, 323–422.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|