


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 21-11-2020
Date: 4-11-2020
Date: 7-11-2020
|
Catalytic Triad
The term catalytic triad is used to describe the arrangement of amino acid residues in the active sites of serine proteinases that underlies their mechanism of enzyme action. It was first applied to the active site of chymotrypsin when the structure of that enzyme was determined by X-ray crystallography (1). Earlier studies had shown that chymotrypsin could be inactivated by chemical modification of one of its serine residues with the nerve gas, diisopropylfluorophosphate (DIFP). Examination of the enzyme structure revealed that the hydroxyl group of this serine, residue 195, was “activated” by the imidazole group of histidine 57, which in turn was “activated” by the carboxyl group of aspartate 102 (Fig. 1). Originally described as a charge relay system, these three residues are more commonly called a catalytic triad.
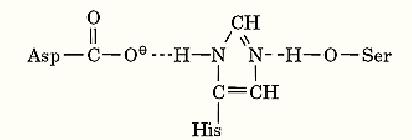
Figure 1. The catalytic triad, the hydrolytic apparatus of serine proteinases. It consists of the side chains of an aspartic acid, a histidine, and a serine residue (eg, residues 102, 57 and 195, respectively, in bovine chymotrypsin). In the absence of substrate, the imidazole group of the histidine is unprotonated, but it potentiates the nucleophilic properties of the hydroxyl side chain of the serine. When substrate binds to the active site, the serine proton is transferred to the imidazole group and the oxygen attacks the carbonyl group of the substrate. The resulting positively charged imidazole is stabilized by interaction with the negative charge of the aspartate carboxyl group. The activated serine is also highly reactive toward inhibitors such as PMSF and DIFP.
Similar triads have been observed in both the chymotrypsin and subtilisin evolutionary families of serine proteinases (2), as well as in other hydrolytic enzymes (3). It is remarkable that the bacterial serine proteinase subtilisin has the same geometrical arrangement of aspartate, histidine, and serine residues as chymotrypsin, but all other structural aspects of the two proteins are quite different. This is a classic example of convergent evolution. The activated serine interacts with the carbonyl carbon atom of the peptide bond to be hydrolyzed, forming an oxyanion intermediate, which is converted to an acylated serine with release of the amine component of the peptide. Subsequent hydrolysis of the acylenzyme occurs by the reverse reaction in which water substitutes for the activated serine.
References
1. D. M. Blow (1976) Acc. Chem. Res. 9, 145–152.
2. T. A. Steitz and R. G. Schulman (1982) Annu. Rev. Biochem. Biophys. 11, 419–444.
3. D. M. Blow (1990) Nature 343, 694–695.



|
|
|
|
مخاطر خفية لمكون شائع في مشروبات الطاقة والمكملات الغذائية
|
|
|
|
|
|
|
"آبل" تشغّل نظامها الجديد للذكاء الاصطناعي على أجهزتها
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|