


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 19-11-2015
Date: 18-11-2015
Date: 18-11-2015
|
Animal Virus Infection
1. Definitions
1.1. Viruses
Supramolecular structures made of nucleic acid (the viral genome), proteins, glycosylated or not, and, eventually (in the case of enveloped viruses), lipids. Viruses are obligatory parasites of cells. They use the cell machinery of protein biosynthesis to make enzymes and regulatory and structural proteins. In some cases, they also use cellular polymerases to synthesize their nucleic acids. The lipid-bilayer, the coat of enveloped viruses, is of cellular origin.
1.2. Infection
Invasion of a cell or an organism by an agent that, most of the time, will propagate, kill the host cell, and eventually cause disease.
2. Classification of Viruses
The International Committee on Taxonomy of Viruses (1) (ICTV) has proposed adopting a universal system of virus taxonomy. The system uses hierarchical levels such as order (suffix -virales), family (suffix -viridae), subfamily (suffix -virinae), genus (suffix -virus) and species (”a virus species is defined as a polythetic class of viruses that constitutes a replicating lineage and occupies a particular ecological niche”). It should be recognized that great difficulties are being encountered in deciding whether a particular virus should be designated as a species or as a subspecies or strain or variant. The properties of viruses used in taxonomy are properties of the virion (morphology, physicochemical, and physical properties) and of the viral proteins, carbohydrates, lipids, and antigenic and biologic properties. The ICTV was recognizing more than 3,600 virus species and hypothesizing that more than 30,000 viruses, virus strains, and subtypes were being tracked in reference centers and culture collections (1). The ultimate goal is to catalogue “data down to subspecies, strain, variant, and isolate levels, that is, levels important in medicine, agriculture, and other scholarly fields.” Obviously, the task is gigantic. Its achievement will require major investments in biochemical and biophysical techniques, as well as in bioinformatics. The 1995 state of the art has been updated and amplified (2).
This classification takes no account of the diseases caused by viruses. Indeed, it is more and more often observed that many viruses cause no, or only occasional, disease. They have reached a state of harmony with their host; an example is the case of the African green monkey (AGM) strain of simian immunodeficiency virus (SIV), which is very frequently found in the wild with no induction of disease.
Families of viruses infecting invertebrates or vertebrates are illustrated on Figure 1 as virus diagrams. Taxa containing double-stranded (ds) or single-stranded (ss) DNA or RNA (either the + or– strands) are separated by frames. Nonenveloped viruses are depicted as icosahedra. A dotted or hairy outer layer identifies enveloped viruses. A simple classification of viruses, based on how viral messenger RNA is produced, was proposed by D. Baltimore (3) (Fig. 2).

Figure 1. Families of viruses infecting invertebrates and vertebrates. Frames separate taxa containing double-stranded (ds) and single-stranded (ss) genomes. Horizontal blocks separate taxa containing DNA and RNA viruses. Taxa containing reverse transcribing (RT) viruses and the negative (–) and positive (+) ss RNA genomes are also indicated. A dotted or hairy outer layer indicates an enveloped virus. Icosahedral structures designate nonenveloped viruses .
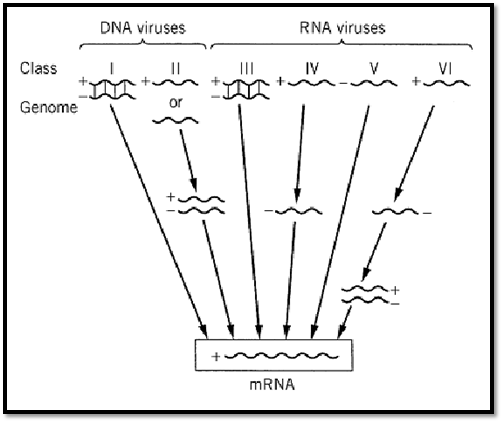
Figure 2. Baltimore's simplified classification of viruses. This classification is based on the type of nucleic acid in the genome and the mode of production of mRNA (3). Class I: Viruses with a ds DNA genome; mRNA is transcribed by the classical mechanism of transcription of a ds DNA template. Class II: Viruses with a ss DNA genome; the DNA strand has the same, positive sense as the mRNA, or else the sense of the genomic strand is negative, opposite to the mRNA. Synthesis of viral mRNA does not proceed before the ss DNA genomes of class II viruses are converted to a ds molecule. Class III: Viruses with ds RNA genome; such genomes are segmented. Transcription of mRNA is catalyzed by an RNA-dependent RNA polymerase packaged in the virion. Class IV: Viruses with an ss RNA genome of the same polarity as mRNA; an ss RNA (–) template is made before mRNA synthesis. Class V: Viruses with an ss RNA (–) genome complementary to the mRNA. Virion RNA is transcribed into mRNA by virion-associated enzymes. Class VI: Retroviruses have a diploid RNA genome made of two molecules of RNA (+). The + sense RNA is used as template by reverse transcriptase for the synthesis of a ds provirus to be integrated in the host DNA; transcription is catalyzed by a cellular DNA-dependent RNA polymerase .

Figure 3. Structure of viruses. (a) A helical virus. The wavy red line represents RNA protected within successive arrays of proteins. (b, c) Spherical viruses. Icosahedral capsids have vertices made of symmetrically organized pentons. Hexons make the faces and are arranged along the edges. (From Ref. 19, with permission). (d) The fivefold, threefold and twofold symmetry axes of a regular icosahedron.
3. Structure of Virions
Figure 1 presents simple diagrams of the structure of virions. Two capsid structures exist; they are either multimers of protein subunits arranged as icosahedra symmetry (quasi-spherical volumes with an external molecular sheet built of 20 identical faces, each of which is an equilateral triangle) or multiple copies of protein subunits arranged as helices. Icosahedral structures can form independently of the presence of nucleic acid, whereas the helical arrangements generally require the presence of nucleic acid to assemble.
The detailed structure of picornaviridae capsids has been determined by X-ray crystallography. Models of other virus capsids at 28 to 35 Å resolution were provided using cryo-electron microscopy and computer single-particle reconstruction methods (rotaviridae, orbiviridae).
Models of helical and icosahedral viruses are illustrated in Fig. 3. Helical viruses largely expose protein subunits, and nucleic acid is protected within (Fig. 3a). Figure 3b, 3c, and 3d illustrate icosahedral structures with 20 triangular faces (each being made of three identical or equivalent capsid subunits), 12 vertices (where five subunits make contact symmetrically) and 30 edges (along which the protein subunits exhibit 6-fold symmetry, as well as the subunits making up the surfaces in between). Although the shape of the 20 faces is not a flat triangle as illustrated in Figure 3 (b and c), the overall appearance of these solids is that of nearly spherical volumes with triangular faces. At each vertex, edge, or face, symmetry is n = 5, 2, and 3, respectively (Fig. 3d).
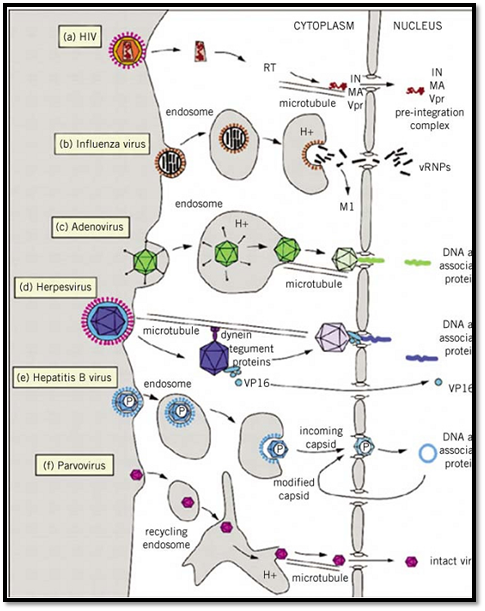
Figure 4. Virus import into the nucleus.
The capsid structure of retroviruses corresponds to the above description. It contains an external matrix protein (a key factor in nuclear targeting) underlying the lipid bilayer and an internal capsid protein, limiting the internal cavity, which contains two identical (+) viral RNA molecules associated with the nucleocapsid protein, the reverse transcriptase, and other enzymes of cellular origin. Originally, retroviruses were classified according to their morphological appearance and budding phenotype as observed by electron microscopy (4).
4. Virus Attachment, Entry, Uncoating, and Transport
Viruses enter cells by interaction with their receptor; human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) use two receptors (it is often spoken of as one receptor, CD4, and one coreceptor, which belongs to the family of receptors for chemokines).
Adenoviridae also use two receptors. The presence of the receptor to a given virus does not ipso facto mean that the cell will be permissive to that virus. Nonreceptor genetic controls of early events in infection have been amply documented in mice, chickens, hamsters, and so on. Receptors can be proteins, sugars, polysaccharides, ceramides, and so on, present at the cell surface. Virus-receptor attachment rates have been measured on a variety of systems. Some happen to be very fast, with a second-order rate constants of about 10–7 cm 3 min–1, others are much slower, 10–12 cm3min–1.
Two major mechanisms of virus entry have been recognized: virus-cell fusion at the plasma membrane, which is pH-independent, and virus endocytosis, which is pH-dependent. Passage of viral structures through a lipid bilayer probably implies extrusion of hydrophobic peptide sequences, as suggested for poliovirus (5). In the case of fusogenic enveloped viruses, the fusogenic peptide at the amino-terminus of the F protein inserts obliquely in the lipid bilayer of the target cell and destabilizes it (6). Virus-cell fusion occurs at the plasma membrane for pH-independent viruses (eg, retroviruses) or in endosomes for pH-dependent viruses (eg, influenza virus) (7).
Enveloped viruses recognize their target cell via interaction of their external envelope protein with a cell membrane protein acting as receptor. The cases of HIV and SIV are more complex because the virus requires both a receptor and a coreceptor for entry. The most commonly used receptor is CD4; the coreceptor is a chemokine receptor. Induction of a conformational change by interaction of HIV-1 external glycoprotein (gp120) with the CD4 receptor was demonstrated by the crystal structure at 2.5 Å resolution of an HIV-1 gp120 core complexed with a two-domain fragment of human CD4 and an antigen-binding fragment of a neutralizing antibody that blocks chemokine-receptor binding (8). The structure revealed a cavity-laden CD4-gp120 interface and a conserved binding site for the chemokine receptor.
Virus-cell fusion implies a major contribution by the viral transmembrane moiety of the envelope glycoprotein. In pH-dependent viruses (ie, influenza virus), each subunit of the trimeric envelope gets rearranged and reorganized largely as an a helix, the fusion peptide (located at the NH2 end of the transmembrane protein, HA2 in this case) being cast some 100 Å outwards towards the target membrane (9). X-ray diffraction studies applied to HIV-1 transmembrane protein gp41 and to Moloney murine leukemia virus illustrate the great similarity between pH-dependent and pH-independent envelope transmembrane protein architectures, suggesting a common mechanism for initiating fusion (9-11).
Virion interaction with the virus receptor(s) leads to release in the cytoplasm of the viral capsid or of the viral nucleic acid. Many aspects of uncoating are still undefined, even in thoroughly studied systems such as poliovirus or HIV. In poliovirus, preinfection maturation of the particle involves loss of protein VP4 and swelling of the particle, and, at very low frequency of a few percent, the stage of an infectosome is supposed to deliver the RNA genome through the membrane and into the cytosol. There is a receptor-mediated extrusion of a hydrophobic structure into the host cell membrane. In this system, the uncoating steps occur outside the cell, with the particle being attached to the receptor (a member of the immunoglobulin family) and in close contact with the cell membrane (Ref. 7, p. 633).
Virus nuclear import (12, 13) is illustrated in Figure 4 and summarized in Table 1. Engulfed in an endosome, adenovirus capsid external protein causes disruption of the endosomal membrane. Freed in the cytosol, the capsid interacts with the nuclear pore complex through a penton structure at one vertex. Viral DNA and associated proteins penetrate the nucleus, leaving behind the empty capsid. Similar mechanisms mediate the transport of herpesvirus capsids to the nucleus. This enveloped virus fuses at the plasma membrane; the capsid moves to the nucleus along microtubules. The capsid-nuclear pore interaction involves one vertex. DNA and associated proteins penetrate the nucleus.
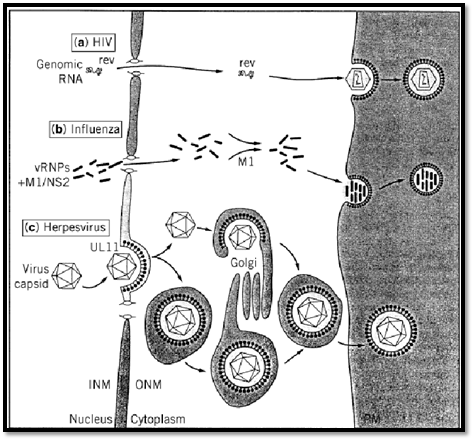
Figure 5. Export of viral material from the nucleus to the cytoplasm and the extracellular compartment. The HIV Rev protein is required for export from the nucleus of full-size and singly spliced viral RNA. An immature capsid gets progressively organized underneath the lipid bilayer of the plasma membrane where viral envelope glycoproteins are concentrated. An analogous process leads to budding of influenza virus particles. Fully assembled herpes virus capsids bud from the inner nuclear membrane and fuse with the outer nuclear membrane, being released naked into the cytoplasm. Naked particles become enwrapped by membranes of the Golgi apparatus and accumulate within a vesicle. Alternatively, the capsid buds through the double membrane of the nucleus, and the outer membrane fuses with a membrane of the Golgi apparatus. The final outcome of this pathway is again an enveloped virus within a cytoplasmic vesicle. Enveloped particles will be released from the cell through fusion of the vesicular membrane with the plasma membrane of the infected cell.
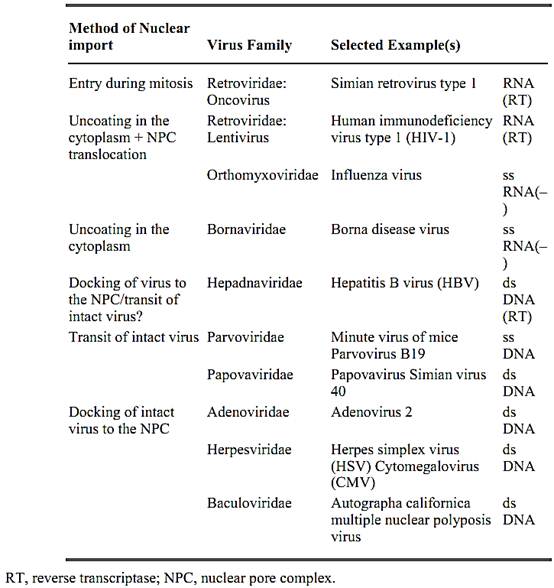
Table 1. Summary of Viruses That Enter the Nucleus
Influenza viruses enter cells by receptor-mediated endocytosis. Uncoating of the virus in endosomes depends on the acidic pH of this compartment. It is supposed that the low pH-activated ion-channel activity of the virion-associated M2 protein permits the flow of ions (essentially protons) inside the virus particle, disrupting of protein-protein interactions and, thus, freeing the ribonucleoproteins (NPs). The low pH also induces a major change in the three-dimensional structure of the transmembrane protein HA2 (see above). The viral envelope fuses with the membrane of the endosome, releasing NPs in the cytosol from where they will move to the nucleus.
Uncoating and transport of retroviral capsids delivered in the cytosol after virus-cell membrane fusion are poorly understood. Reverse transcription, initiated in the producing cell, proceeds during the transport to the nucleus, where integration of the proviral DNA will take place, catalyzed by the viral integrase. Onco-retroviruses depend on the breakdown of the nuclear envelope during mitosis to achieve DNA integration and completion of their life cycle. On the contrary, lentiviruses use the nuclear localization signal of the matrix protein to establish infection in macrophages and quiescent T-lymphocytes (15). In addition to the matrix protein, reverse transcriptase and integrase, the nucleocapsid protein also plays a role by potentiating provirus integration in the presence of Mg2+.
5. Virus Replication
The strategies of viral nucleic acid replication depend on the nature and strandedness of the viral genetic material.
5.1. Double-Stranded DNA Viruses
These viruses, Picornaviridae, replicate in the nucleus. DNA duplication can be carried out by either cellular or viral DNA polymerases. Poxviridae are exceptions. Their multiplication is cytoplasmic. The pox genome encodes the viral DNA polymerase. In single-stranded DNA viruses (Parvoridiae), DNA synthesis takes place in the nucleus, uses cellular DNA polymerases, and derives from a self-priming mechanism. The replicative intermediate is a linear duplex molecule. Virions carry either a (+) or a (–) DNA strand. Hepadnaviridae have a peculiar genomic structure. One strand of DNA (negative sense, complementary to the viral messenger RNAs) is full-length; the other is of variable size. Before transcription by the host RNA polymerase II, the DNA is converted into a covalent closed circle. The closed-circular DNA species can serve as template for the synthesis of a full size mRNA that will serve as a template for synthesis of the minus-strand DNA by reverse transcription using a protein primer.
5.2. Double-Stranded RNA Viruses
These viruses, Reoviridae, carry a genetic material made of ds RNA segments (10 to 16 segments, according to the genus) and a virion-associated RNA-dependent RNA polymerase (or transcriptase). Transcription products of the double-stranded genome are RNA (+) molecules that are either used as mRNA or encapsulated in nascent virions, where they are used as templates to generate a double-stranded genome.
5.3. Single-Stranded RNA (–) Viruses
These viruses, Rhabdoviridae, harbor a negative-sense RNA molecule (11 kb to 15 kb in size) and a virion-associated transcriptase. Replication takes place in the cytoplasm. Host protein factors are required for replication. Replicative intermediates are made of RNA (+) molecules linked to a so-called N protein. In turn, RNA (+) molecules are used as templates for new genomic (–) strands. Segmented negative-strand RNA viruses (ie, orthomyxoviridae [influenza viruses]) have a genome size of 10 kb to 13.6 kb, made of six or seven segments, depending on the genus. At infection, viral nucleocapsids are transported to the cell nucleus, where the virion trancriptase synthesizes mRNA species, which act as templates for new viral RNA synthesis. The latter RNA (–) molecules exist as nucleocapsids in the nucleus of infected cells.
5.4. Single-Stranded RNA (+) Viruses
Virions of single-stranded (+) RNA virus (7 kb to 8.5 kb in size for picornaviruses) contain one molecule of infectious, positive-sense ss RNA. Translation of the genomic RNA generates a polyprotein, which is processed by a viral protease, giving rise to structural virus proteins and nonstructural ones, among which are one subunit of replicase (RNA-dependent RNA polymerase). The replicase probably associates with cellular proteins to make up a functional RNA polymerase. This situation is analogous to that of the Qb phage, in which the virus-encoded replicase subunit associates with elongation factors Tu and Ts and ribosomal protein S1, to build up a highly active replicase to which the virus contributed minimally. Replication first produces a negative-sense RNA, complementary to the RNA (+) genomic RNA. Due to the higher affinity of replicase for the 3′ end sequence of minus strands, many more RNA (+) molecules than RNA (–) molecules are produced, which are either translated or encapsidated in progeny virus.
Retroviridae have a diploid, single-stranded RNA (+) genome and a virion-associated reverse trancriptase (RNA-dependent DNA polymerase). Reverse transcription starts in the virus particle and produces a linear ds DNA copy of the genomic RNA. The linear DNA is integrated into the host cell DNA via the activity of a virus-coded integrase and is transcribed as regular cellular genes. Viruses of that family are oncogenic through their site of integration (activation of proto-oncogenes); other members of the family have incorporated and mutated proto-oncogenes and, consequently, become acute transforming viruses; others (such as human T-cell leukemia virus) are transforming entities through dysregulation of the cell transcription machinery. The lentivirinae are primarily inducers of immunodeficiency.
6. Synthesis of Viral Messenger RNA and Viral Proteins
6.1. Double-stranded DNA viruses (ie, Papovaviridae)
The life cycle of many viruses can be divided into an early and a late period. In ds DNA viruses, proteins made in the first period are called early; they play regulatory functions. Proteins made later in the life cycle of the virus are structural molecules. Precursor mRNAs undergo classical post-transcriptional processing, including 5′-capping, polyadenylation, and RNA splicing. Efficient use of the available information involves alternative splicing and use of overlapping open reading frames. A peculiarity seen in Poxviridae, adenoviridae, and some families of parvoviridae is the transcription of early proteins from both strands of the ds genome; this is another strategy to use maximally the available genomic sequences.
6.2. Double-Stranded RNA Viruses
Viruses of this family carry a virion-associated transcriptase. Transcription of each segment of the ds segmented genome generates an mRNA coding for a single polypeptide chain, a polyprotein.
6.3. Single-Stranded RNA (–) Viruses
The genes of these viruses are transcribed processively, from the 3′ to the 5′ end of the template virus RNA and in decreasing molar abundance. The mRNAs are 5′-capped, 3′-polyadenylated, and generally monocistronic. In the case of viruses with a segmented genome, the largest genome segments encode one protein each, whereas some of the smaller segments code for additional proteins from spliced or bicistronic mRNAs. Influenza virus mRNA synthesis occurs in the nucleus. It requires initiation by host-cell primers and specifically capped RNA fragments derived from host-cell RNA polymerase II transcripts. In this system, viral mRNAs start with cellular sequences (10 to 13 nucleotides).
6.4. Single-Stranded RNA (+) Viruses
A frequent strategy found in this group of viruses is the translation of the + sense genomic RNA into a polyprotein, maturated later on by a virus encoded proteinase (ie, picornaviridae, poliovirus). In alphaviridae (Semliki Forest Virus), the full-length genomic RNA codes for nonstructural proteins. The message for structural proteins is subgenomic and corresponds to the 3′ region of the genomic RNA.
Transcription of retroviral messages occurs from the integrated proviral DNA. It combines all the known mechanisms of eukaryotic transcription. Expression of retroviral information includes expression of regulatory functions from spliced mRNAs, expression of internal structural proteins from genomic, full-length RNA, and expression of envelope proteins from a singly spliced mRNA. Nuclear export of the full-length and singly spliced viral RNA requires interaction with a viral regulatory protein called Rex in Human T-lymphotropic Virus (HTLV) and bovine leukemia virus (BLV). A lentiviral protein with analogous function is called Rev in HIV and SIV.



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|