

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Pathogenesis of Multiple Sclerosis
المؤلف:
Amal Adil Kasid Alsudany
المصدر:
The role of Chemokines (CCL2, CCL5 and CXCL10) in the Neuroinflammatory Among Patients With Multiple Sclerosis -رسالة ماجستير -كلية الطب -جامعة البصرة
الجزء والصفحة:
p12-14
2025-02-13
890
The characteristic pathological hallmark of MS is perivenular inflammatory lesions, leading to demyelinating plaques (Karussis,2014). These plaques which occur due to unknown etiology leading eventually to an inflammatory process with focal destruction of myelin sheath vary in their number, size, distribution and activity (Mahad et al., 2015).
Many white matter lesions are detected in specific predilection sites, notably around the ventricles. Other predilection sites include the optic nerve and spinal cord. Moreover, lesions may also occur in the deep white matter far from ventricles and grey matter (Lassmann et al., 2007).
The white matter lesions can be characterized broadly as chronic and active lesions. In chronic lesions, there are fewer mononuclear cells, almost complete demyelination, and severe astrogliosis. Active lesions are defined by the ongoing destruction of myelin and are heavily infiltrated by macrophages and microglial cells (Frohman et al., 2006).
Clinical and Magnetic resonance imaging (MRI) data suggest that inflammation and formation of new white matter lesions are the substrates for Relapsing remitting multiple sclerosis (RRMS). In the progressive phase, the new inflammatory demyelinating lesions are rare. Still, diffuse atrophy of the grey and white matter and changes in the normal-appearing white matter (NAWM) are more predominant (Lassmann et al., 2007). Although MS is considered an inflammatory demyelinating disease of the CNS, axonal injury and loss also occur in the MS lesions. They are associated with developing permanent disability in MS patients (Bjartmar et al., 2010).
This suggests that progressive axonal loss may induce the transition from Relapsing remitting multiple sclerosis (RRMS) to Secondary Progressive multiple sclerosis (SPMS). Axon pathology and the frequency of transected axons in MS lesions correlate with the degree of inflammation (Frischer et al., 2009).
Despite a clinical distinction between RRMS and progressive MS, pathologically -defined inflammatory changes are seen in both, albeit to a greater degree in relapsing -remitting disease. The composition of the inflammatory infiltrate in relapsing -remitting and progressive MS is similar, although the proportion of B -cells and plasma cells is higher in progressive MS (Dobson & Giovannoni, 2019).
Myelin is not limited to white matter, and demyelination in MS also involves gray matter, especially in the cerebral cortex (Pedre et al., 2011). Cortical lesions have been observed in 80% of patients with Primary Progressive multiple sclerosis (PPMS). These cortical lesions histopathological characteristics differ substantially from white matter lesions (Calabrese et al., 2010).
Three types of cortical lesions have been reported, two of these lesions may appear in continuity with subcortical white matter plaques (type I) or as small intracortical perivascular lesions (type II). The most abundant form of cortical demyelination is subpial demyelination, which appears as large band-like lesions extending from the cortex's outer surface into its deeper layer (type III) (Peterson et al., 2001).
Lesions in the cortex are less inflammatory than those in the white matter, and the blood-brain barrier is far less permeable to fluids. (Absinta et al., 2016). Disruption of the optic nerve due to multiple sclerosis (CNS) and loss of adjacent retinal ganglion cells are both commonly seen in the disease. Thinning of the retina's nerve fiber and ganglion-cell layers are visualized using optical coherence tomography. Thinning occurs when the axons of the optic nerve are injured. (Petzold et al., 2017), as seen in figure (1).
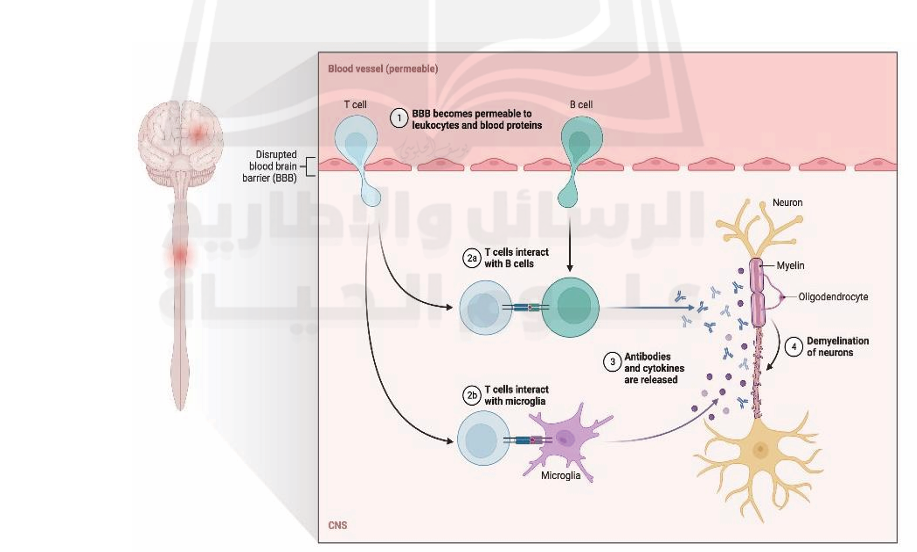
Fig1. pathogenesis of multiple sclerosis. (https://app.biorender.com/biorender-templates/t-5fb3ee3a6abae900abef1965-pathogenesis-of multiple-sclerosis )
References
----------------
Absinta, M., Sati, P., and Reich, D. S. (2016). Advanced MRI and staging of multiple sclerosis lesions. Nature Reviews Neurology, 12(6), 358-368.
Bjartmar, L., Alkhori, L., Ruud, J., Mohammed, A. H., Marcusson, J., and Hallbeck, M. (2010). Long-term treatment with antidepressants, but not environmental stimulation, induces expression of NP2 mRNA in hippocampus and medial habenula. Brain research, 1328, 25-33.
Calabrese, M., Filippi, M., and Gallo, P. (2010). Cortical lesions in multiple sclerosis. Nature Reviews Neurology, 6(8), 438-444.
Dobson, R., and Giovannoni, G. (2019). Multiple sclerosis–a review. Europ. J. neurol., 26(1), 27-40.
Frischer, J. M., Bramow, S., Dal-Bianco, A., Lucchinetti, C. F., Rauschka, H., Schmidbauer, M., ... and Lassmann, H. (2009). The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain, 132(5), 1175-1189.
Frohman, E. M., Racke, M. K., and Raine, C. S. (2006). Multiple sclerosis—the plaque and its pathogenesis. New Eng. J. Med., 354(9), 942 955.
Karussis, D. (2014). The diagnosis of multiple sclerosis and the various related demyelinating syndromes: a critical review. Journal of autoimmunity, 48, 134-142.
Lassmann, H., Brück, W., and Lucchinetti, C. F. (2007). The immunopathology of multiple sclerosis: an overview. Brain pathology, 17(2), 210-218.
Mahad, D. H., Trapp, B. D., and Lassmann, H. (2015). Pathological mechanisms in progressive multiple sclerosis. The Lancet Neurology, 14(2), 183-193.
Pedre, X., Mastronardi, F., Bruck, W., López-Rodas, G., Kuhlmann, T., and Casaccia, P. (2011). Changed histone acetylation patterns in normal appearing white matter and early multiple sclerosis lesions. Journal of Neuroscience, 31(9), 3435-3445.
Peterson, J. W., Bö, L., Mörk, S., Chang, A., and Trapp, B. D. (2001). Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 50(3), 389-400.
Petzold, A., Balcer, L. J., Calabresi, P. A., Costello, F., Frohman, T. C., Frohman, E. M., Martinez-Lapiscina, E. H., Green, A. J., Kardon, R., Outteryck, O., and others. (2017). Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. The Lancet Neurology, 16(10), 797-812.
 الاكثر قراءة في الامراض المناعية
الاكثر قراءة في الامراض المناعية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















