


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 28-9-2020
Date: 4-10-2020
Date: 20-12-2015
|
Addition of hydrogen to a multiple bond is hydrogenation. It is applicable to almost all types of multiple bonds and is of great importance in synthetic chemistry, particularly in the chemical industry. Probably the most important technical example is production of ammonia by the hydrogenation of nitrogen:
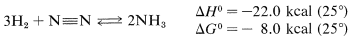
This may appear to be a simple process, but in fact it is difficult to carry out because the equilibrium is not very favorable. High pressures (150-200 atm) are required to get a reasonable conversion, and high temperatures (430-510o) are necessary to get reasonable reaction rates. A catalyst, usually iron oxide, also is required. The reaction is very important because ammonia is used in ever-increasing amounts as a fertilizer either directly or through conversion to urea or ammonium salts.
Production of ammonia requires large quantities of hydrogen, most of which comes from the partial oxidation of hydrocarbons with water or oxygen. A simple and important example is the so-called "methane-steam gas" reaction, which is favorable only at very high temperatures because of the entropy effect in the formation of H2 :
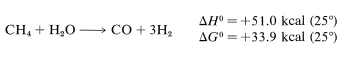
Therefore the fertilizer industry is allied closely with the natural gas and petroleum industries, and for obvious reasons ammonia and hydrogen often are produced at the same locations.,
Alkenes and alkynes add hydrogen much more readily than does nitrogen. For example, ethene reacts rapidly and completely with hydrogen at ordinary pressures and temperatures in the presence of metal catalysts such as nickel, platinum, palladium, copper, and chromium:
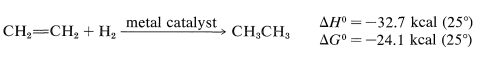
These reactions are unlike any we have encountered so far. They are heterogeneous reactions, which means that the reacting system consists of two or more phases. Usually, the metal catalyst is present as a finely divided solid suspension in the liquid or solution to be reduced. Alternatively, the metal is deposited on an inert solid support such as carbon, barium sulfate, alumina (Al2O3), or calcium carbonate. Then the mixture of the liquid substrate and solid catalyst is shaken or stirred in a hydrogen atmosphere. However, then actual reaction takes place at the surface of the metal catalyst and is an example of heterogeneous or surface catalysis.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
قسم التربية والتعليم يكرّم الطلبة الأوائل في المراحل المنتهية
|
|
|