
Radical-Chain Addition Reactions to Alkenes
 المؤلف:
John D. Roberts and Marjorie C. Caserio
المؤلف:
John D. Roberts and Marjorie C. Caserio
 المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
 الجزء والصفحة:
........
الجزء والصفحة:
........
 19-1-2022
19-1-2022
 7062
7062
Radical-Chain Addition Reactions to Alkenes
The early literature concerning the addition of hydrogen bromide to unsymmetrical alkenes at best is confused. Sometimes the same alkene was reported to give addition both according to, and in opposition to, the principles discussed for electrophilic ionic addition . Much of the uncertainty on the addition of hydrogen bromide was removed by the classical researches of M. S. Kharasch and F. R. Mayo (1933) who showed that there must be two reaction mechanisms, each giving a different product. Kharasch and Mayo found, in the presence of radical inhibitors, hydrogen bromide adds to propene in a rather slow reaction to give pure 2-bromopropane:

With light, peroxides, radical initiators, and in the absence of radical inhibitors a rapid radical-chain addition of hydrogen bromide occurs to yield 80% or more of 1-bromopropane:
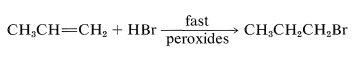
Similar effects have been noted occasionally with hydrogen chloride, but never with hydrogen iodide or hydrogen fluoride. A few substances apparently add to alkenes only by radical mechanisms, and always add in the opposite way to that expected for electrophilic ionic addition.
Two questions with regard to the so-called abnormal addition will be given special attention. Why does the radical mechanism give a product of different structure than the ionic addition? Why does the radical addition occur readily with hydrogen bromide but rarely with the other hydrogen halides?
The abnormal addition of hydrogen bromide is catalyzed strongly by peroxides, which have the structure R−O−O−R and decompose thermally to give RO⋅ radicals:

The RO⋅ radicals can react with hydrogen bromide in two ways, to abstract either hydrogen atoms or bromine atoms:
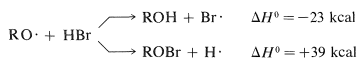
Clearly, the formation of ROH and a bromine atom is energetically more favorable. The overall process of decomposition of peroxide and attack on hydrogen bromide, which results in the formation of a bromine atom, can initiate a radical-chain addition of hydrogen bromide to an alkene.
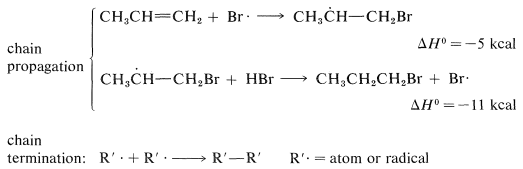
The two chain-propagating steps, taken together, are exothermic by 16kcal and have a fairly reasonable energy balance between the separate steps. The reaction chains apparently are rather long, because the addition is strongly inhibited by radical traps and only traces of peroxide catalyst are needed.
 الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


