

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Hydration of Alkynes
المؤلف:
John D. Roberts and Marjorie C. Caserio
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
الجزء والصفحة:
........
18-1-2022
3175
Hydration of Alkynes
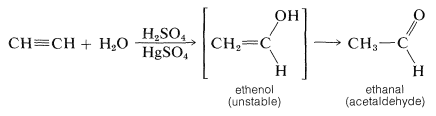
Alkynes, unlike alkenes, are not hydrated readily in aqueous acid unless a mercuric salt is present as a catalyst. Also, the products that are isolated are either aldehydes or ketones instead of alcohols. Even though the addition of one molecule of water to ethyne probably gives ethenol (vinyl alcohol) initially, this compound is unstable relative to its structural isomer (ethanal) and rapidly rearranges:
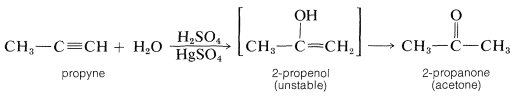
Similarly, addition of water to propyne leads to 2-propanone by way of its unstable isomer, 2-propenol:
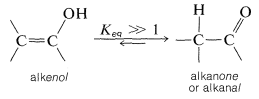
In general, the position of equilibrium for interconversion of a carbonyl compound with the corresponding alkenol (or enol), having the hydroxyl group attached to the double bond, lies far on the side of the carbonyl compound:
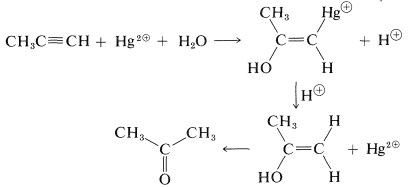
Because mercuric salts catalyze the hydration of alkynes, they probably are acting as electrophiles. Mercuric salts are known to add to both alkenes and alkynes, and if the reaction mixture is acidic, the carbon-mercury bond is cleaved to form a carbon-hydrogen bond. The overall sequence in propyne hydration may be written as follows:
 الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















