


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 6-1-2022
التاريخ: 2024-07-12
التاريخ: 6-10-2020
التاريخ: 14-12-2020
|
Many important molecules such as ammonia, water, and hydrogen fluoride have atoms with unshared pairs of electrons:
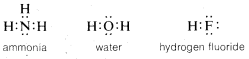
If we formulate each of these molecules in such a way to minimize repulsions between like charges, a basically tetrahedral arrangement will be expected because this will place the nuclei (and electron pairs) as widely separated as possible.
Figure 6-11: Abbreviated atomic-orbital model of ethane showing only the orbitals of the outer-shell electrons
The water molecule could be formulated this way, as in 6, with the oxygen at the center of the tetrahedron:
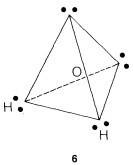
The simple picture predicts that the H−O−H bond angle should be tetrahedral, 109.5o. But actually it is 104.5o.
There are two schools of thought as to why the angle is 104.5o. One idea is that the repulsion model is too simple and has to be modified to take into account that the repulsion is more severe between pairs of unshared electrons than between electrons in bonding orbitals on the same atom. This is because when a bond is formed between two nuclei, the attraction of the nuclei for the electrons shrinks the orbitals available to the bonding electrons, thereby reducing their electrostatic repulsion with other pairs. The degree of repulsion between electron pairs diminishes in the sequence: unshared pairs vs. unshared pairs > unshared pairs vs. bonding pairs > bonding pairs vs. bonding pairs. From this, we expect that in water the H−O−H angle will be less than tetrahedral, because the larger repulsion between the two unshared pairs will tend to push the bonding pairs closer together.

A similar, but smaller, effect is expected for ammonia because now the repulsion is only between the one unshared pair and bonding pairs. The ammonia H−N−H angle is 107.3o, which is only slightly smaller than the tetrahedral value of 109.5o.
The alternative point of view of why the bond angle of water is 104.5o starts with the premise that, in the simplest approximation, the angle should be 90o90o! To see how this comes about let us compare H:Be:H with H:O:H You will recall that to form two bonds to BeBe, we had to promote an electron and change the electronic configuration to the valence configuration, (2s)1(2p)1. The situation with H2O is different in that the oxygen ground state and valence state are the same, (2s)2(2px)1(2py)1(2pz)1. This means we could form two equivalent bonds to oxygen using the 2py and 2pz orbitals at an angle of 90o (Figure 6-12).
Figure 6-12: Overlap of the hydrogen 1s1s orbitals with y2py and 2pz orbitals centered on oxygen. The p orbitals are represented here with distorted shapes to make the drawing clearer.
Now, to explain why the H−O−H bond angles are 104.5o instead of 90o, we can say that the repulsion between the hydrogen nuclei is expected to widen the bond angle. An argument in favor of this formulation is provided by the bond angle in H2S, which is 92.2o. This is much closer to the 90o90o expected for p-bond orbitals and the hydrogens in H2S would not be expected to repel each other as much as in H2O because sulfur is a larger atom than oxygen.
Both ways of formulating the orbitals used in the bonding of water molecules are in current use. Arguments can be advanced in favor of both. Highly sophisticated quantum-mechanical calculations, which we will say more about later, suggest that oxygen in water molecules uses orbitals that are 18%s and 82%p in its bonds (sp4.5), and furthermore, that the unshared pairs are in equivalent hybrid orbitals [not one pair as (2s)2 and the other as (2p)2]. Each of the unshared electron-pair orbitals of oxygen in water is calculated to be about 40%s and 60%p (sp1.5).
The results are hardly clearcut, but the bonding orbitals are considerably closer to sp3 (25%s and 75%p) than they are to 100%p. We recommend that the bonding orbitals of nitrogen and oxygen be considered to be sp3 and the unshared pairs designated simply as (n)2. An abbreviated atomic orbital model of methanol, CH3OH, made on this basis is shown in Figure 6-13.
Figure 6-13: Abbreviated atomic-orbital model of methanol, CH3OH, showing the orbitals of the outer-shell electrons only



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|