

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
The D, L Convention for Designating Stereochemical Configurations
المؤلف:
John D. Roberts and Marjorie C. Caserio
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
الجزء والصفحة:
........
28-12-2021
2580
The D, L Convention for Designating Stereochemical Configurations
It is equally important to be able to unambiguously describe the configuration of a compound. The convention that is used to designate the configurations of chiral carbons of naturally occurring compounds is called the D,L system. To use it, we view the molecule of interest according to the following rules:
1. The main carbon chain is oriented vertically with the lowest numbered carbon at the top. The numbering used for this purpose must follow the IUPAC rules:
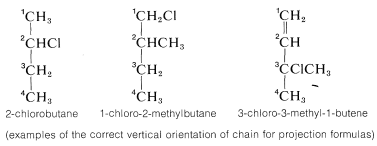
2. Next, the structure must be arranged at the particular chiral carbon whose configuration is to be assigned so the horizontal bonds to that carbon extend toward you and the vertical bonds extend away from you. This arrangement will be seen to be precisely the same as the convention of projection formulas such as 5c and 6c.
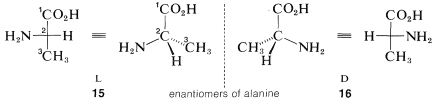
3. Now the relative positions of the substituents on the horizontal bonds at the chiral centers are examined. If the main substituent is the left of the main chain, the L configuration is assigned; if this substituent is on the right, the D configuration is assigned.
For example, the two configurations of the amino acid, alanine, would be represented in perspective or projection as 15 and 16. The carboxyl carbon is C1 and is placed at the top. The substituents at the chiral carbon connected to the horizontal bonds are amino (−NH2) and hydrogen. The amino substituent is taken to be the main substituent; when this is on the left the acid has the LL configuration, and when it is on the right, the D configuration. All of the amino acids that occur in natural proteins have been shown to have the L configuration.
Glyceraldehyde, CH2OHCHOHCHO, which has one chiral carbon bonded to an aldehyde function, hydrogen, hydroxyl, and hydroxymethyl (CH2OH ), is of special interest as the simplest chiral prototype of sugars (carbohydrates). Perspective views and Fischer projections of the D and LL forms correspond to 17 and 18 , respectively, where the carbon of the aldehyde function (−CH=O ) is C1 :
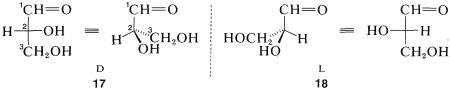
The D,L system of designating configuration only can be applied when there is a main chain, and when we can make an unambiguous choice of the main substituent groups. Try, for instance, to assign D and L configurations to enantiomers of bromochlorofluoromethane. An excellent set of rules has been worked out for such cases that leads to unambiguous configurational assignments by what is called the R,S convention.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















