
Practical Halogenations and Problems of Selectivity
 المؤلف:
John D. Roberts and Marjorie C. Caserio
المؤلف:
John D. Roberts and Marjorie C. Caserio
 المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
 الجزء والصفحة:
........
الجزء والصفحة:
........
 25-12-2021
25-12-2021
 2115
2115
Practical Halogenations and Problems of Selectivity
Given the knowledge that a particular reaction will proceed at a suitable rate, a host of practical considerations are necessary for satisfactory operation. These considerations include interference by possible side reactions that give products other than those desired, the ease of separation of the desired product from the reaction mixture, and costs of materials, apparatus, and labor. We shall consider these problems in connection with the important synthetic reactions discussed in this book.
The chlorination of saturated hydrocarbons can be induced by light, but also can be carried out at temperatures of about 300o in the dark. Under such circumstances the mechanism is similar to that of light-induced chlorination, except that the chlorine atoms are formed by thermal dissociation of chlorine molecules. Solid carbon surfaces catalyze thermal chlorination, possibly by aiding in the cleavage of the chlorine molecules.
Direct monohalogenation of saturated hydrocarbons works satisfactorily only with chlorine and bromine. For the general reaction
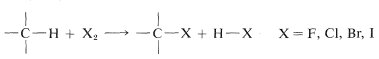
the calculated ΔH0ΔH0 value is negative and very large for fluorine, negative and moderate for chlorine and bromine, and positive for iodine (see Table 4-7). With fluorine, the reaction evolves so much heat that it may be difficult to control, and products from cleavage of carbon-carbon as well as of carbon-hydrogen bonds may be obtained. The only successful, direct fluorination procedure for hydrocarbons involves diffusion of minute amounts of fluorine mixed with helium into liquid or solid hydrocarbons at low temperatures, typically −78o (Dry Ice temperature). As fluorination proceeds, the concentration of fluorine can be increased. The process is best suited for preparation of completely fluorinated compounds, and it has been possible to obtain in this way amounts of (CF3)4C and (CF3)3C−C(CF3)3 from 2,2-dimethylpropane and 2,2,3,3-tetramethylbutane corresponding to 10-15% yields based on the fluorine used.
Bromine generally is much less reactive toward hydrocarbons than chlorine is, both at high temperatures and with activation by light. Nonetheless, it usually is possible to brominate saturated hydrocarbons successfully. Iodine is unreactive.
Table 4-7: Calculated Heat of Reaction for Halogenation fo Hydrocarbons
| Halogen (X) |
ΔHoΔHo (kcal/mole)a |
| F |
-116 |
| Cl |
-27 |
| Br |
-10 |
| I |
13 |
| |
|
The chlorination of methane does not have to stop with the formation of chloromethane (methyl chloride). It is usual when chlorinating methane to obtain some of the higher chlorination products: dichloromethane (methylene chloride), trichloromethane (chloroform), and tetrachloromethane (carbon tetrachloride):
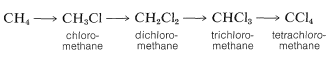
In practice, one can control the degree of substitution to a considerable extent by controlling the methane-chlorine ratio. For example, for monochlorination to predominate, a high methane-chlorine ratio is necessary such that the chlorine atoms react with CH4 and not with CH3Cl.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


