


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 5-10-2021
Date: 24-8-2021
Date: 14-11-2021
|
Glycolysis Reactions
The conversion of glucose to pyruvate occurs in two stages (Fig. 1). The first five reactions of glycolysis correspond to an energy-investment phase in which the phosphorylated forms of intermediates are synthesized at the expense of ATP. The subsequent reactions of glycolysis constitute an energy-generation phase in which a net of two molecules of ATP are formed by substrate-level phosphorylation per glucose molecule metabolized.

Figure 1: Two phases of aerobic glycolysis. NAD(H) = nicotinamide adenine dinucleotide; ADP = adenosine diphosphate.
A. Glucose phosphorylation
Phosphorylated sugar molecules do not readily penetrate cell membranes because there are no specific transmembrane carriers for these compounds and because they are too polar to diffuse through the lipid core of membranes. Therefore, the irreversible phosphorylation of glucose (Fig. 2) effectively traps the sugar as cytosolic glucose 6-phosphate and commits it to further metabolism in the cell. Mammals have four isozymes (I–IV) of the enzyme hexokinase that catalyze the phosphorylation of glucose to glucose 6-phosphate.
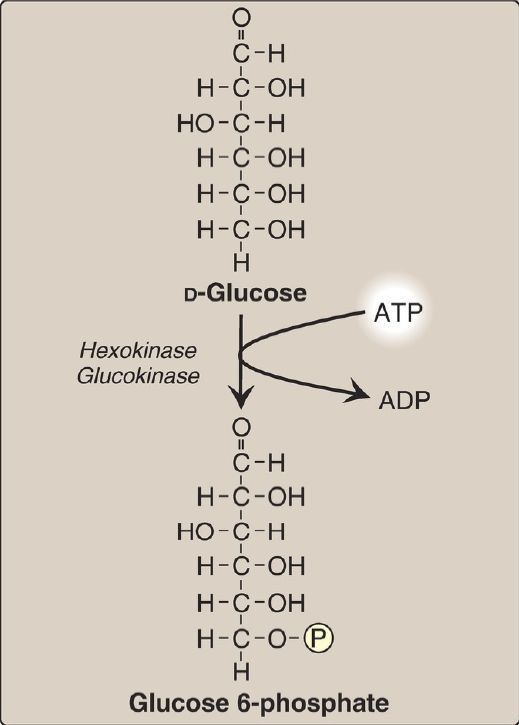
Figure 2: Energy-investment phase: phosphorylation of glucose. [Note: Kinases utilize ATP complexed with a divalent metal ion, most typically magnesium.] ADP = adenosine diphosphate; P = phosphate.
1. Hexokinases I–III: In most tissues, glucose phosphorylation is catalyzed by one of these isozymes of hexokinase, which is one of three regulatory enzymes of glycolysis (along with phosphofructokinase and pyruvate kinase). They are inhibited by the reaction product glucose 6-phosphate, which accumulates when further metabolism of this hexose phosphate is reduced. Hexokinases I–III have a low Michaelis constant (Km) and, therefore, a high affinity for glucose. This permits the efficient phosphorylation and subsequent metabolism of glucose even when tissue concentrations of glucose are low (Fig. 3). However, because these isozymes have a low maximal velocity ([Vmax] ) for glucose, they do not sequester (trap) cellular phosphate in the form of phosphorylated glucose or phosphorylate more glucose than the cell can use. [Note: These isozymes have broad substrate specificity and are able to phosphorylate several hexoses in addition to glucose.]
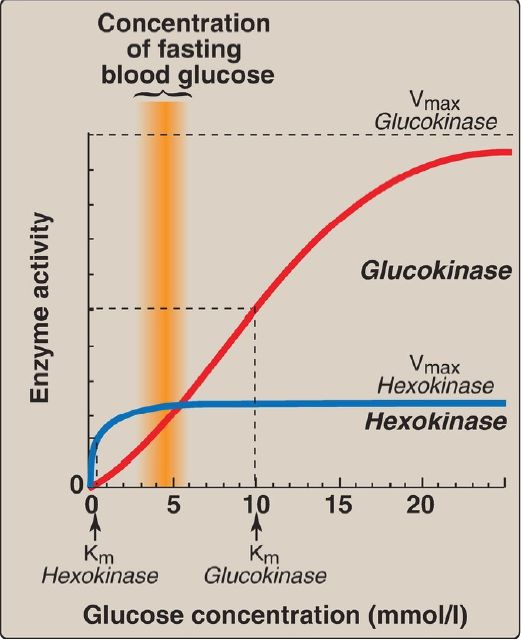
Figure 3: Effect of glucose concentration on the rate of phosphorylation catalyzed by hexokinase and glucokinase. Km = Michaelis constant; Vmax = maximal velocity.
2. Hexokinase IV: In liver parenchymal cells and pancreatic β cells, glucokinase (the hexokinase IV isozyme) is the predominant enzyme responsible for glucose phosphorylation. In β cells, glucokinase functions as a glucose sensor, determining the threshold for insulin secretion . [Note: Hexokinase IV also serves as a glucose sensor in hypothalamic neurons, playing a key role in the adrenergic response to hypoglycemia .] In the liver, the enzyme facilitates glucose phosphorylation during hyperglycemia. Despite the popular but misleading name glucokinase, the sugar specificity of the enzyme is similar to that of other hexokinase isozymes.
a. Kinetics: Glucokinase differs from hexokinases I–III in several important properties. For example, it has a much higher Km, requiring a higher glucose concentration for half-saturation (see Fig. 3).Thus, glucokinase functions only when the intracellular concentration of glucose in the hepatocyte is elevated such as during the brief period following consumption of a carbohydrate-rich meal, when high levels of glucose are delivered to the liver via the portal vein. Glucokinase has a high Vmax, allowing the liver to effectively remove the flood of glucose delivered by the portal blood. This prevents large amounts of glucose from entering the systemic circulation following such a meal, thereby minimizing hyperglycemia during the absorptive period. [Note: GLUT-2 insures that blood glucose equilibrates rapidly across the hepatocyte membrane.]
b. Regulation: Glucokinase activity is not directly inhibited by glucose 6-phosphate as are the other hexokinases. Instead, it is indirectly inhibited by fructose 6-phosphate (which is in equilibrium with glucose 6-phosphate, a product of glucokinase) and is indirectly stimulated by glucose (a substrate of glucokinase). Regulation is achieved by reversible binding to the hepatic protein glucokinase regulatory protein (GKRP). In the presence of fructose 6-phosphate, glucokinase binds tightly to GKRP and is translocated to the nucleus, thereby rendering the enzyme inactive (Fig. 4). When glucose levels in the blood (and also in the hepatocyte, as a result of GLUT-2) increase, glucokinase is released from GKRP, and the enzyme reenters the cytosol where it phosphorylates glucose to glucose 6-phosphate. [Note: GKRP is a competitive inhibitor of glucose use by glucokinase.]

Figure 4: Regulation of glucokinase activity by glucokinase regulatory protein. GLUT = glucose transporter.
Glucokinase functions as a glucose sensor in blood glucose homeostasis. Inactivating mutations of glucokinase are the cause of a rare form of diabetes, maturity onset diabetes of the young type 2 (MODY 2) that is characterized by impaired insulin secretion and hyperglycemia.
B. Glucose 6-phosphate isomerization
The isomerization of glucose 6-phosphate to fructose 6-phosphate is catalyzed by phosphoglucose isomerase (Fig. 5). The reaction is readily reversible and is not a rate-limiting or regulated step.
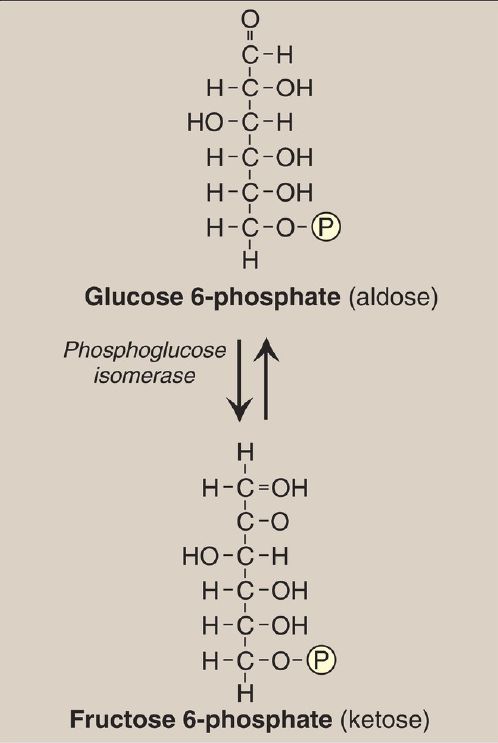
Figure 5: Aldose-ketose isomerization of glucose 6-phosphate to fructose 6-phosphate. P = phosphate.
C. Fructose 6-phosphate phosphorylation
The irreversible phosphorylation reaction catalyzed by phosphofructokinase-1 (PFK-1) is the most important control point and the rate-limiting and committed step of glycolysis (Fig. 6). PFK-1 is controlled by the available concentrations of the substrates ATP and fructose 6-phosphate as well as by other regulatory molecules.

Figure 6: Energy-investment phase (continued): conversion of fructose 6-phosphate to triose phosphates. P = phosphate; AMP and ADP = adenosine mono- and diphosphates.
1. Regulation by intracellular energy levels: PFK-1 is inhibited allosterically by elevated levels of ATP, which act as an energy-rich signal indicating an abundance of high-energy compounds. Elevated levels of citrate, an intermediate in the TCA cycle , also inhibit PFK-1. [Note: Inhibition by citrate favors the use of glucose for glycogen synthesis .] Conversely, PFK-1 is activated allosterically by high concentrations of AMP, which signal that the cell’s energy stores are depleted.
2. Regulation by fructose 2,6-bisphosphate: Fructose 2,6-bisphosphate is the most potent activator of PFK-1 (see Fig. 6) and is able to activate the enzyme even when ATP levels are high. It is formed from fructose 6-phosphate by phosphofructokinase-2 (PFK-2). Unlike PFK-1, PFK-2 is a bifunctional protein that has both the kinase activity that produces fructose 2,6-bisphosphate and the phosphatase activity that dephosphorylates fructose 2,6-bisphosphate to fructose 6-phosphate. In the liver isozyme, phosphorylation of PFK-2 inactivates the kinase domain and activates the phosphatase domain (Fig. 7). The opposite is seen in the cardiac isozyme. Skeletal PFK-2 is not covalently regulated. [Note: Fructose 2,6-bisphosphate is an inhibitor of fructose 1,6-bisphosphatase, an enzyme of gluconeogenesis . The reciprocal actions of fructose 2,6-bisphosphate on glycolysis (activation) and gluconeogenesis (inhibition) insure that both pathways are not fully active at the same time, preventing a futile cycle of glucose oxidation to pyruvate followed by glucose resynthesis from pyruvate.]
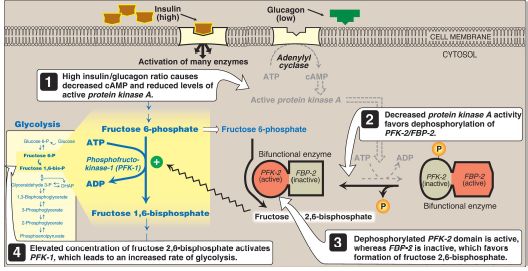
Figure 7: Effect of elevated insulin concentration on the intracellular concentration of fructose 2,6-bisphosphate in the liver. PFK-2 = phosphofructokinase-2; FBP-2 = fructose 2,6-bisphosphatase; AMP and ADP = adenosine mono- and diphosphates; cAMP = cyclic AMP; = phosphate.
a. During the well-fed state: Decreased levels of glucagon and elevated levels of insulin (such as occur following a carbohydrate-rich meal) cause an increase in hepatic fructose 2,6-bisphos- phate (PFK-2 is dephosphorylated) and, thus, in the rate of glycolysis (see Fig. 7). Therefore, fructose 2,6-bisphosphate acts as an intracellular signal of glucose abundance.
b. During fasting: By contrast, the elevated levels of glucagon and low levels of insulin that occur during fasting cause a decrease in hepatic fructose 2,6-bisphosphate (PFK-2 is phosphorylated). This results in inhibition of glycolysis and activation of gluconeogenesis.
D. Fructose 1,6-bisphosphate cleavage
Aldolase cleaves fructose 1,6-bisphosphate to dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (see Fig. 6). The reaction is reversible and not regulated. [Note: Aldolase B, the hepatic isoform, also cleaves fructose 1-phosphate and functions in dietary fructose metabolism .]
E. Dihydroxyacetone phosphate isomerization
Triose phosphate isomerase interconverts DHAP and glyceraldehyde 3-phosphate (see Fig. 6). DHAP must be isomerized to glyceraldehyde 3-phosphate for further metabolism by the glycolytic pathway. This isomerization results in the net production of two molecules of glyceraldehyde 3-phosphate from the cleavage products of fructose 1,6-bisphosphate. [Note: DHAP is utilized in triacylglycerol synthesis .]
F. Glyceraldehyde 3-phosphate oxidation
The conversion of glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate (1,3-BPG) by glyceraldehyde 3-phosphate dehydrogenase is the first oxidation-reduction reaction of glycolysis (Fig. 8). [Note: Because there is a limited amount of NAD+ in the cell, the NADH formed by the dehydrogenase reaction must be oxidized for glycolysis to continue. Two major mechanisms for oxidizing NADH to NAD+ are the reduction of pyruvate to lactate by lactate dehydrogenase (LDH) and the electron transport chain ([ETC] aerobic ). Because NADH cannot cross the inner mitochondrial membrane, the ETC requires the malate-aspartate and glycerol 3-phosphate substrate shuttles to move NADH reducing equivalents into the mitochondrial matrix .]
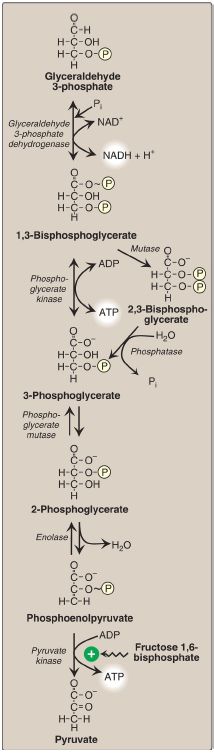
Figure 8: Energy-generating phase: conversion of glyceraldehyde 3-phosphate to pyruvate. NAD(H) = nicotinamide adenine dinucleotide; P = phosphate; Pi =inorganic phosphate; ~ = high-energy bond; ADP = adenosine diphosphate.
1. 1,3-Bisphosphoglycerate synthesis: The oxidation of the aldehyde group of glyceraldehyde 3-phosphate to a carboxyl group is coupled to the attachment of Pi to the carboxyl group. This phosphate group, linked to carbon 1 of the 1,3-BPG product by a high-energy bond , conserves much of the free energy produced by the oxidation of glyceraldehyde 3-phosphate. This high-energy phosphate drives ATP synthesis in the next reaction of glycolysis.
2. Arsenic poisoning: The toxicity of arsenic is due primarily to the inhibition by trivalent arsenic (arsenite) of enzymes such as the pyruvate dehydrogenase complex (PDHC), which require lipoic acid as a coenzyme. However, pentavalent arsenic (arsenate) can prevent net ATP and NADH production by glycolysis without inhibiting the pathway itself. It does so by competing with Pi as a substrate for glyceraldehyde 3-phosphate dehydrogenase, forming a complex that spontaneously hydrolyzes to form 3-phosphoglycerate (see Fig. 8). By bypassing the synthesis of and phosphate transfer from 1,3-BPG, the cell is deprived of energy usually obtained from the glycolytic pathway.[Note: Arsenate also competes with Pi binding to the F1 domain of ATP synthase , resulting in formation of ADP-arsenate that is rapidly hydrolyzed.]
3. 2,3-Bisphosphoglycerate synthesis in RBC: Some of the 1,3-BPG is converted to 2,3-BPG by the action of bisphosphoglycerate mutase (see Fig. 8). 2,3-BPG, which is found in only trace amounts in most cells, is present at high concentration in RBC and serves to increase O2 delivery . 2,3-BPG is hydrolyzed by a phosphatase to 3-phosphoglycerate, which is also an intermediate in glycolysis (see Fig. 8). In the RBC, glycolysis is modified by inclusion of these shunt reactions.
G. 3-Phosphoglycerate synthesis and ATP production
When 1,3-BPG is converted to 3-phosphoglycerate, the high-energy phosphate group of 1,3-BPG is used to synthesize ATP from ADP (see Fig.8). This reaction is catalyzed by phosphoglycerate kinase, which, unlike most other kinases, is physiologically reversible. Because two molecules of 1,3-BPG are formed from each glucose molecule, this kinase reaction replaces the two ATP molecules consumed by the earlier formation of glucose 6-phosphate and fructose 1,6-bisphosphate. [Note: This reaction is an example of substrate-level phosphorylation, in which the energy needed for the production of a high-energy phosphate comes from a substrate rather than from the ETC .]
H. Phosphate group shift
The shift of the phosphate group from carbon 3 to carbon 2 of phosphoglycerate by phosphoglycerate mutase is freely reversible.
I. 2-Phosphoglycerate dehydration
The dehydration of 2-phosphoglycerate by enolase redistributes the energy within the substrate, forming phosphoenolpyruvate (PEP), which contains a high-energy enol phosphate (see Fig. 8). The reaction is reversible, despite the high-energy nature of the product. [Note: Fluoride inhibits enolase, and water fluoridation reduces lactate production by mouth bacteria, decreasing dental caries ]



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|