
Biosynthesis of Collagen
 المؤلف:
Denise R. Ferrier
المؤلف:
Denise R. Ferrier
 المصدر:
Lippincott Illustrated Reviews: Biochemistry
المصدر:
Lippincott Illustrated Reviews: Biochemistry
 الجزء والصفحة:
الجزء والصفحة:
 3-9-2021
3-9-2021
 1536
1536
Biosynthesis of Collagen
The polypeptide precursors of the collagen molecule are synthesized in fibroblasts (or in the related osteoblasts of bone and chondroblasts of cartilage). They are enzymically modified and form the triple helix, which gets secreted into the ECM. After additional enzymic modification, the mature extracellular collagen fibrils aggregate and become cross-linked to form collagen fibers.
1. Pro-α chain formation: Collagen is one of many proteins that normally function outside of cells. Like most proteins produced for export, the newly synthesized polypeptide precursors of α chains (prepro-α chains) contain a special amino acid sequence at their amino (N)-terminal ends.
This sequence acts as a signal that, in the absence of additional signals, targets the polypeptide being synthesized for secretion from the cell. The signal sequence facilitates the binding of ribosomes to the rough endoplasmic reticulum (RER) and directs the passage of the prepro-α chain into the lumen of the RER. The signal sequence is rapidly cleaved in the lumen to yield a precursor of collagen called a pro-α chain .
2. Hydroxylation: The pro-α chains are processed by a number of enzymic steps within the lumen of the RER while the polypeptides are still being synthesized . Proline and lysine residues found in the Yposition of the –Gly–X–Y– sequence can be hydroxylated to form hydroxyproline and hydroxylysine residues. These hydroxylation reactions require molecular oxygen, ferrous iron (Fe2+), and the reducing agent vitamin C (ascorbic acid), without which the hydroxylating enzymes, prolyl hydroxylase and lysyl hydroxylase, are unable to function . In the case of ascorbic acid deficiency (and, therefore, a lack of proline and lysine hydroxylation), interchain Hbond formation is impaired, as is formation of a stable triple helix.
Additionally, collagen fibrils cannot be cross-linked, greatly decreasing the tensile strength of the assembled fiber. The resulting deficiency disease is known as scurvy. Patients with scurvy often show ecchymoses (bruise-like discolorations) on the limbs as a result of subcutaneous extravasation (leakage) of blood due to capillary fragility (Fig. 1).
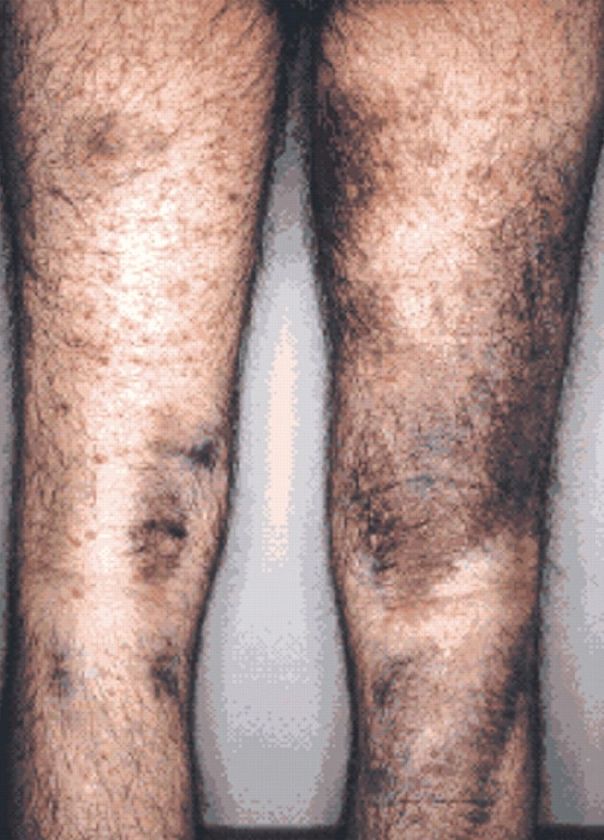
Figure 1: The legs of a 46-year-old man with scurvy.
3. Glycosylation: Some hydroxylysine residues are modified by glycosylation with glucose or glucosyl-galactose .
4. Assembly and secretion: After hydroxylation and glycosylation, three pro-α chains form procollagen, a precursor of collagen that has a central region of triple helix flanked by the nonhelical N- and carboxyl (C)-terminal extensions called propeptides . The formation of procollagen begins with formation of interchain disulfide bonds between the C-terminal extensions of the pro-α chains. This brings the three α chains into an alignment favorable for triple helix formation. The procollagen molecules move through the Golgi apparatus, where they are packaged in secretory vesicles. The vesicles fuse with the cell membrane, causing the release of procollagen molecules into the extracellular space.
5. Extracellular cleavage of procollagen molecules: After their release, the triple-helical procollagen molecules are cleaved by N- and Cprocollagen peptidases, which remove the terminal propeptides, producing tropocollagen molecules.
6. Collagen fibril formation: Tropocollagen molecules spontaneously associate to form collagen fibrils. They form an ordered, parallel array, with adjacent collagen molecules arranged in a staggered pattern, each overlapping its neighbor by a length approximately three quarters of a molecule .
7. Cross-link formation: The fibrillar array of collagen molecules serves as a substrate for lysyl oxidase. This copper-containing extracellular enzyme oxidatively deaminates some of the lysine and hydroxylysine residues in collagen. The reactive aldehydes that result (allysine and hydroxyallysine) can spontaneously condense with lysine or hydroxylysine residues in neighboring collagen molecules to form covalent cross-links and, thus, mature collagen fibers (Fig. 2). [Note:Cross-links can form between two allysine residues.]

Figure 2: Formation of cross-links in collagen. [Note: Lysyl oxidase is irreversibly inhibited by a toxin present in seeds from Lathyrus odoratus (sweet pea), leading to a condition known as lathyrism that is characterized by skeletal and vascular problems.] Cu2+ = copper; NH3 = ammonia; H2O2 = hydrogen peroxide.
Lysyl oxidase is one of several copper-containing enzymes. Others include ceruloplasmin , cytochrome c oxidase , dopamine hydroxylase , superoxide dismutase , and tyrosinase . Disruption in copper homeostasis causes copper deficiency (X-linked Menkes syndrome) or overload (Wilson disease) .
 الاكثر قراءة في الكيمياء الحيوية
الاكثر قراءة في الكيمياء الحيوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


