

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
The Unfolded Protein Response Is Related to tRNA Splicing
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المصدر:
LEWIN’S GENES XII
الجزء والصفحة:
17-5-2021
1913
The Unfolded Protein Response Is Related to tRNA Splicing
KEY CONCEPTS
- Ire1 is an inner nuclear membrane protein with its Nterminal domain in the ER lumen and its C-terminal domain in the nucleus; the C-terminal domain exhibits both kinase and endonuclease activities.
- Binding of an unfolded protein to the N-terminal domain activates the C-terminal endonuclease by autophosphorylation.
- The activated endonuclease cleaves HAC1 (Xbp1 in vertebrates) mRNA to release an intron and generate exons that are ligated by a tRNA ligase.
- Only spliced HAC1 mRNA can be translated to a transcription factor that activates genes encoding chaperones that help to fold unfolded proteins.
- Activated Ire1 induces apoptosis when the cell is overstressed by unfolded proteins.
An unusual splicing system that is related to tRNA splicing is the unfolded protein response (UPR) pathway conserved in eukaryotes. As summarized in FIGURE 1, the accumulation of unfolded proteins in the lumen of the endoplasmic reticulum (ER) triggers the UPR pathway. This leads to increased transcription of genes encoding chaperones that assist protein folding in the ER. A signal must therefore be transmitted from the lumen of the ER to the nucleus.
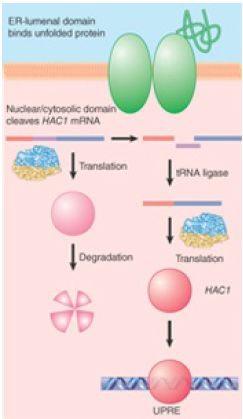
FIGURE 1.The unfolded protein response occurs by activating special splicing of HAC1 mRNA to produce a transcription factor that recognizes the UPRE.
The sensor that activates the pathway is the inositol-requiring protein Ire1, which is localized in the ER and/or inner nuclear membrane. The N-terminal domain of Ire1 lies in the lumen of the ER where it detects the presence of unfolded proteins, presumably by binding to exposed motifs. The C-terminal half of Ire1 is located in either the cytoplasm or nucleus (because of the continuous membrane of the ER and the nucleus) and exhibits both Ser/Thr kinase activity and a specific endonuclease activity. Binding of unfolded proteins causes aggregation of Ire1 monomers on the ER membrane, leading to the activation of the C-terminal domain on the other side of the membrane by autophosphorylation.
The activated C-terminal endonuclease has, at present, only one (though important) substrate, which is the mRNA encoding the UPR-specific transcription factor Hac1 in yeast (Xbp1 in vertebrates). Under normal conditions, when the UPR pathway is not activated, HAC1 mRNA contains a 252-nucleotide intron (Xbp1 contains a 26-nucleotide intron). The intron in HAC1 prevents the mRNA from being translated into a functional protein in yeast, whereas in mammalian cells the intron in Xbp1 allows translation, but the protein is rapidly degraded by the proteosome. Unusual splicing components are involved in processing this intron. The activated Ire1 endonuclease acts directly on HAC1 mRNA (Xbp1 mRNA in vertebrates) to cleave the two splicing junctions, leaving 2′,3′-cyclic phosphate at the 3′ end of the 5′ exon and 5′–OH at the 5′ end of the 3′ exon. The two junctions are then ligated by the tRNA ligase that acts in the tRNA-splicing pathway. Thus, the entire pathway for processing HAC1 (Xbp1) pre-mRNA resembles the pre-tRNA pathway.
Important differences exist between the two pathways, however. Ire1 and tRNA endonuclease share no sequence homology or subunit composition. The endonuclease activity of IreI is highly regulated in the ER and has only one substrate (HAC1 pre-mRNA). In contrast, tRNA endonuclease has many substrates, all with common tRNA folding, with little preference for sequences surrounding the splice sites.
By using such a tRNA-like pathway to remove the intron in the HAC1 (Xbp1) mRNA, the mature mRNA can be translated to produce a potent basic-leucine zipper (bZIP) transcription factor to bind to a common motif (UPRE) in the promoter of many downstream genes. The gene products protect the cell by increasing the expression of proteins to assist protein folding.
If the UPR system is overwhelmed by unfolded proteins, the activated kinase domain of Ire1 binds to the TRAF2 adaptor molecule in the cytoplasm to activate the apoptosis pathway and kill the cell. Thus, the cell uses an unusual tRNA-processing strategy to respond to unfolded proteins. However, there is no apparent relationship between the Ire1 endonuclease and the tRNA-splicing endonuclease, so it is not obvious how this specialized system would have evolved.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















