

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Nonreplicative Transposition Proceeds by Breakage and Reunion
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المصدر:
LEWIN’S GENES XII
الجزء والصفحة:
22-4-2021
2660
Nonreplicative Transposition Proceeds by Breakage and Reunion
KEY CONCEPTS
- Nonreplicative transposition results if a crossover structure is nicked on the unbroken pair of donor strands and the target strands on either side of the transposon are ligated.
- The two pathways for nonreplicative transposition differ according to whether the first pair of transposon strands are joined to the target before the second pair are cut (Tn5), or whether all four strands are cut before joining to the target (Tn10).
The crossover structure can also be used in nonreplicative transposition. The principle of nonreplicative transposition by this mechanism is that a breakage and reunion reaction allows the target to be reconstructed with the insertion of the transposon; the donor remains broken. No cointegrate is formed.
FIGURE 1shows the cleavage events that generate nonreplicative transposition of phage Mu. Once the unbroken donor strands have been nicked, the target strands on either side of the transposon can be ligated. The single-stranded regions generated by the staggered cuts must be filled in by repair synthesis. The product of this reaction is a target replicon in which the transposon has been inserted between repeats of the sequence created by the original single-strand nicks. The donor replicon has a double-strand break across the site where the transposon was originally located.

FIGURE 1.Nonreplicative transposition results when a crossover structure is released by nicking. This inserts the transposon into the target DNA, flanked by the direct repeats of the target, and the donor is left with a double-strand break.
Nonreplicative transposition can also occur by an alternative pathway in which nicks are made in target DNA, but a doublestrand break is made on either side of the transposon, releasing it entirely from flanking donor sequences . This cut-and-paste pathway is used by Tn10 and by many eukaryotic transposons and is illustrated in FIGURE 2.
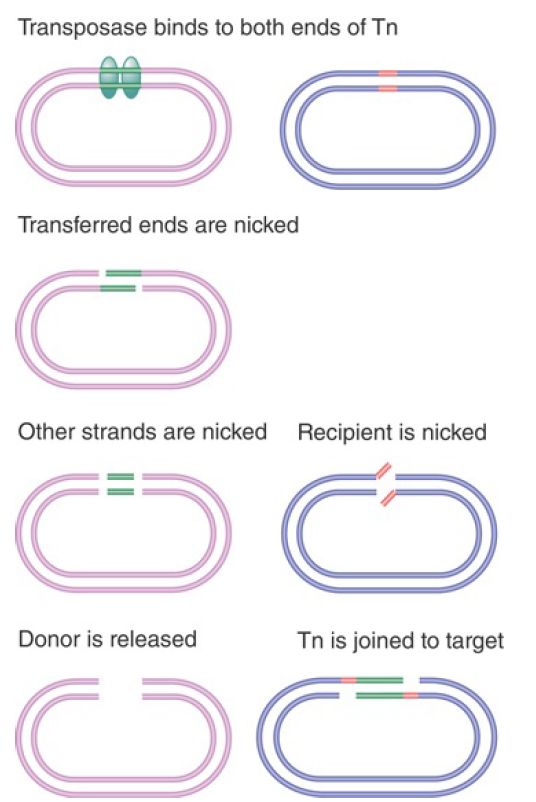
FIGURE 2. Both strands of Tn10 are cleaved sequentially, and then the transposon is joined to the nicked target site.
A simple experiment to prove that Tn10 transposes nonreplicatively made use of an artificially constructed heteroduplex of Tn10 that contained single-base mismatches. If transposition involves replication, the transposon at the new site will contain information from only one of the parent Tn10 strands. If, however, transposition takes place by physical movement of the existing transposon, the mismatches will be conserved at the new site. This proves to be the case.
The basic difference in Figure 1 from the model of Figure 2 is that both strands of Tn10 are cleaved before any connection is made to the target site. The first step in the reaction
is recognition of the transposon ends by the transposase, forming a proteinaceous structure within which the reaction occurs. At each end of the transposon, the strands are cleaved in a specific order: The transferred strand (the one to be connected to the target site) is cleaved first, followed by the other strand. (This is the same order as in the Mu transposition Figure 1).
Tn5 also transposes by nonreplicative transposition. FIGURE 3 shows the interesting cleavage reaction that separates the transposon from the flanking sequences. First, one DNA strand is nicked. The 3′–OH end that is released then attacks the other strand of DNA. This releases the flanking sequence and joins the two strands of the transposon in a hairpin. An activated water molecule then attacks the hairpin to generate free ends for each strand of the transposon.

FIGURE 3. Cleavage of Tn5 from flanking DNA involves nicking, interstrand reaction, and hairpin cleavage.
In the next step, the cleaved donor DNA is released, and the transposon is joined to the nicked ends at the target site. The transposon and the target site remain constrained in the proteinaceous structure created by the transposase (and other proteins). The double-strand cleavage at each end of the transposon precludes any replicative-type transposition and forces the reaction to proceed by nonreplicative transposition, thus giving the same outcome as in Figure 2, but with the individual cleavage and joining steps occurring in a different order. The Tn5 and Tn10 transposases both function as dimers. Each subunit in the dimer has an active site that successively catalyzes the double-strand breakage of the two strands at one end of the transposon, and then catalyzes staggered cleavage of the target site. FIGURE 4 illustrates the structure of the Tn5 transposase bound to the cleaved transposon. Each end of the transposon is located in the active site of one subunit. One end of the subunit also contacts the other end of the transposon. This controls the geometry of the transposition reaction. Each of the active sites will cleave one strand of the target DNA. It is the geometry of the complex that determines the distance between these sites on the two target strands (9 bp in the case of Tn5).

FIGURE 4. Each subunit of the Tn5 transposase has one end of the transposon located in its active site and also makes contact at a different site with the other end of the transposon.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















