


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 21-9-2020
Date: 30-12-2020
Date: 9-12-2020
|
Isothermal Compression
If we shoved the piston back in, from a volume V2 to a volume V1 in Figure (1), we would have to do work on the gas. If we kept the temperature constant, then the pressure would increase along the curve shown in Figure (1) and the work we did would be precisely equal to the area under the curve. In this case work is done on the gas (we could say that during the compression the gas does negative work). When work is done on the gas, the temperature of the gas will rise unless we let heat flow out of the cylinder. Thus if we have an isothermal compression, where there is no increase in the thermal energy of the gas, then we have the pure conversion of useful work into the heat expelled by the piston. This is the opposite of what we want for a power plant.
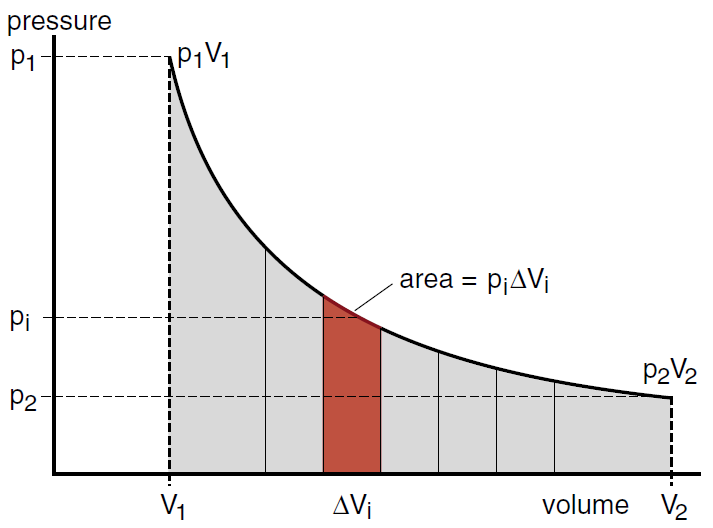
Figure 1: The work done by an expanding gas is equal to the sum of all pΔ V 's , which is the area under the pressure curve.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|