
Introduction to Molecular Engineering of Antibodies
 المؤلف:
John M Walker and Ralph Rapley
المؤلف:
John M Walker and Ralph Rapley
 المصدر:
Molecular Biology and Biotechnology 5th Edition
المصدر:
Molecular Biology and Biotechnology 5th Edition
 الجزء والصفحة:
الجزء والصفحة:
 8-12-2020
8-12-2020
 1745
1745
Introduction to Molecular Engineering of Antibodies
Antibodies are one of the effector molecules of the vertebrate humoral immune system. They are generated in vivo in response to the presence of foreign pathogens or molecules (antigens), bind specifically to the antigen and result in its neutralization and elimination. One of the characteristics of antibodies is that they can bind with high affinity and specificity to only the target antigen and not to any of the tens of thousands of other proteins and potential antigens in the circulation. This specific and high affinity binding led to the appreciation that antibodies could be the socalled ‘magic bullets’ proposed by Ehrlich at the turn of the 20th century: molecules that could selectively target a disease-causing organism and deliver a toxic payload, killing only the organism targeted. As will be described below, antibodies in the serum of immunized animals were some of the first therapeutics used for infectious diseases, at a time before antibiotics had been discovered. Yet it took more than 100 years from this initial use of serum therapy for antibodies to begin to be approved by the US Food and Drug Administration (FDA) for the treatment of human diseases. The first such antibodies entered clinical practice
in the 1990s and today there are currently 21 therapeutic antibodies approved by the FDA which had sales of more than $15 billion in 2006.
Most of these antibodies have been approved for clinical use within the last 10 years and it is estimated that there are at least 100 antibodies in the different phases of human clinical trials for a wide range of diseases, including cancer, inflammatory diseases and infectious diseases.
The era of antibodies as therapeutics became possible with the advent of hybridoma technology in 1975, a technological breakthrough that resulted in the ability to clone single B-cells and the single antibody made by that Bcell (Figure ).Such monoclonal antibodies (mAbs), unlike the hundreds to thousands of antibodies in serum, recognized only a single antigen and could be made in virtually unlimited quantities. Unfortunately, the technology was developed to make mAbs from the B-cells of immunized mice and it has proven technically challenging to apply the technology to generate human mAbs. As it turns out, when murine mAbs are administered to humans, they elicit an immune response, called the human antimouse antibody response (HAMA) that either results in unacceptable systemic reactions or results in rapid clearance of the mAb from the bloodstream. While many mAbs from hybridomas entered clinical trials, few were approved by the FDA due to the limitations described above.
With the advent of molecular cloning and protein engineering technologies in the late 1980s, it has proven possible to engineer murine mAbs to have sequences more similar to human mAbs, so called chimeric8–10 and humanized antibodies. Such antibodies have proven significantly less immunogenic than murine mAbs and many of these are now approved for clinical use. More recently, it has proven possible to make mAbs that are fully human in sequence using antibody gene diversity libraries and display technologies , and also mice that are transgenic for the human immunoglobulin loci.
In the following sections, we will review the increasing importance of antibodies as a therapeutic class and review antibody structure, generation and function. We will use this information as background to describe the molecular engineering techniques of chimerization and humanization that have yielded the first widely successful therapeutic antibodies. We will then describe how the more recent techniques using diversity libraries and display technologies can be used to generate full human antibodies and to evolve antibody affinity to values not typically generated by the humoral immune system.
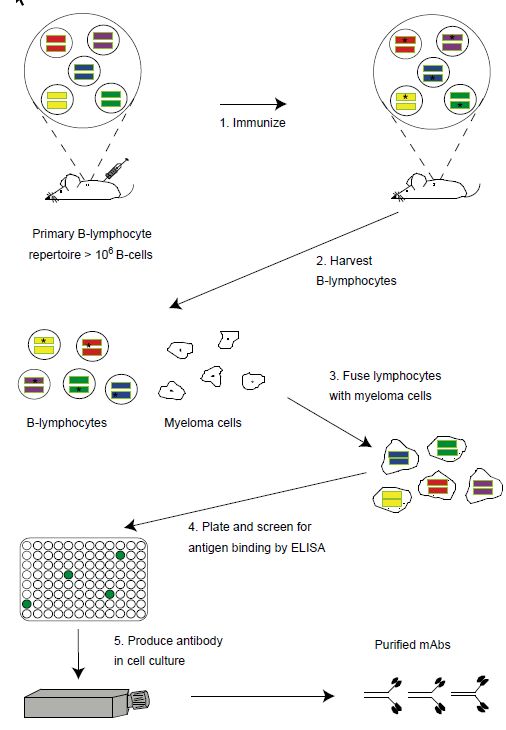
Figure: Generation of monoclonal antibodies using hybridoma technology. The naı¨ve mouse generates a primary repertoire of more than 106 rearranged VH and VL genes (colored bars) in B-cells, coding for antibodies that are displayed as membrane-bound molecules. Immunization (step 1) causes antigen-driven proliferation and somatic hypermutation (‘stars’ within V genes represent mutations introduced by the somatic hypermutation machinery). To make hybridomas, B-cells are harvested from the spleen (step 2) or marrow of the mouse and fused with immortal myeloma cells (wrinkled edged cells, step 3) to generate immortalized, antibody secreting hybridomas. Hybridomas are plated into microtiter plates and the supernatants containing secreted antibody are screened by ELISA for antigen binding (step 4). Hybridomas are expanded into tissue culture flasks and the secreted monoclonal antibodies purified (step 5).
 الاكثر قراءة في المناعة
الاكثر قراءة في المناعة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


