


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 8-3-2016
Date: 7-3-2016
Date: 8-3-2016
|
Light
The view of the hydrogen atom as a miniature solar system, a view of the atom seen through the “lens” of Newtonian mechanics, fails to explain much of the atom’s behavior. When you heat hydrogen gas, it glows with a reddish glow that consists of three distinct colors or so called spectral lines. The colors of the lines are bright red, swimming pool blue, and deep violet. You need more than Newtonian mechanics to understand why hydrogen emits light, let alone explain these three special colors.
In the middle of the 1800s, Michael Faraday went a long way in explaining electric and magnetic phenomena in terms of electric and magnetic fields. These fields are essentially maps of electric and magnetic forces. In 1860 James Clerk Maxwell discovered that the four equations governing the behavior of electric and magnetic fields could be combined to make up what is called a wave equation. Maxwell could construct his wave equation after making a small but crucial correction to one of the underlying equations.
The importance of Maxwell’s wave equation was that it predicted that a particular combination of electric and magnetic fields could travel through space in a wavelike manner. Equally important was the fact that the wave equation allowed Maxwell to calculate what the speed of the wave should be, and the answer was about a billion feet per second. Since only light was known to travel that fast, Maxwell made the guess that he had discovered the theory of light, that light consisted of a wave of electric and magnetic fields of force.
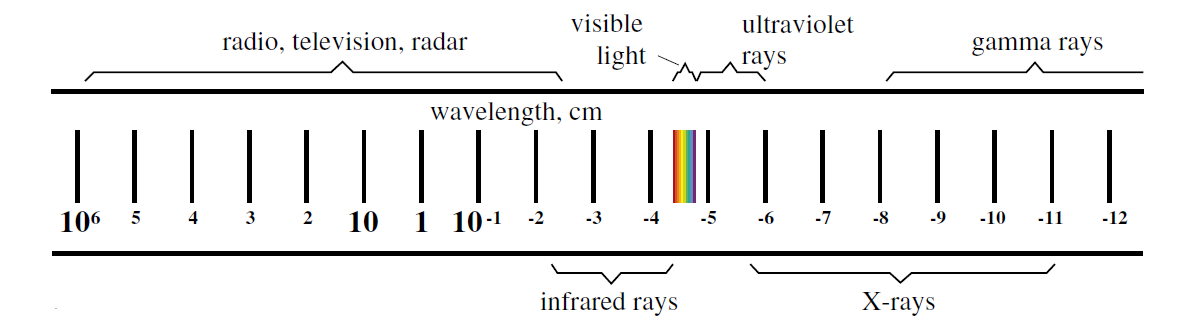
Figure 1: The electromagnetic spectrum.
Visible light is only a small part of what we call the electromagnetic spectrum. Our eyes are sensitive to light waves whose wavelength varies only over a very narrow range. Shorter wavelengths lie in the ultraviolet or x ray region, while at increasingly longer wavelengths are infra red light, microwaves, and radio waves. Maxwell’s theory made it clear that these other wavelengths should exist, and within a few years, radio waves were discovered. The broadcast industry is now dependent on Maxwell’s equations for the design of radio and television transmitters and receivers. (Maxwell’s theory is what is usually taught in the second half of an introductory physics course. That gets you all the way up to 1860.)
While Maxwell’s theory works well for the design of radio antennas, it does not do well in explaining the behavior of a hydrogen atom. When we apply Maxwell’s theory to the miniature solar system model of hydrogen, we do predict that the orbiting electron will radiate light. But we also predict that the atom will self destruct. The unambiguous prediction is that the electron will continue to radiate light of shorter and shorter wavelength while spiraling in faster and faster toward the nucleus, until it crashes. The combination of Newton’s laws and Maxwell’s theory is known as Classical Physics. We can easily see that classical physics fails when applied even to the simplest of atoms.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|