


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-7-2019
Date: 16-10-2020
Date: 23-9-2020
|
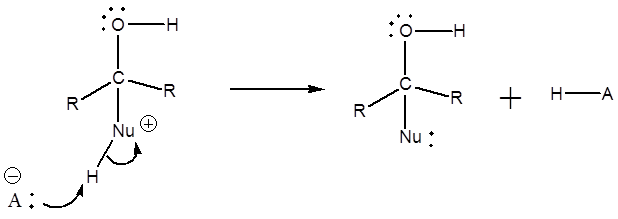
Weak nucleophiles are not strong enough to add to a simple C=O bond. They require the carbon to be made more electrophilic, and this can be done using either a Lewis acid or simply H+. We will focus on activation using simple acid – a process called protonation. This leads to an activated, charged form of the C=O which is now electrophilic enough to react with even weak nucleophiles via nucleophilic addition.
Most often the weak nucleophiles are uncharged compounds such as alcohols (ROH) or amines (RNH2 or similar). With these neutral nucleophiles, the product of the nucleophilic addition step is a positively charged compound, and so there is usually a final acid-base step to remove the unwanted H+ (“deprotonation”) and form the final uncharged product.
Mechanism
1) Protonation of the carbonyl

2) Nucleophilic addition to the activated carbonyl

3) Deprotonation



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|