

 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 29-9-2019
Date: 6-6-2016
Date: 30-10-2019
|
Benzene is resistant to addition reactions. Adding something new to the ring would require that some of the delocalized electrons form bonds with the substituent being added, resulting in a major loss of stability because the delocalization is broken. Instead, benzene primarily undergoes substitution reactions - replacing one or more of the hydrogen atoms with a new substituent, preserving the delocalized electrons as they were.
The reactivity of a compound like methylbenzene must be considered in two distinct parts:
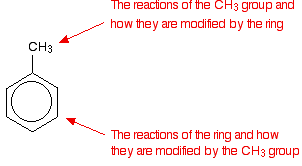
For example, if you explore other pages in this section, you will find that alkyl groups attached to a benzene ring are oxidized by alkaline potassium manganate(VII) solution. This oxidation does not occur in the absence of the benzene ring. The tendency of the CH3 group to "push" electrons away from itself also has an effect on the ring, making methylbenzene react more quickly than benzene itself. You will find this explored in other pages in this section as well.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|