

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Reactions and Compounds of Nitrogen
المؤلف:
LibreTexts Project
المصدر:
................
الجزء والصفحة:
.................
11-6-2020
1792
Reactions and Compounds of Nitrogen
Like carbon, nitrogen has four valence orbitals (one 2s and three 2p), so it can participate in at most four electron-pair bonds by using sp3 hybrid orbitals. Unlike carbon, however, nitrogen does not form long chains because of repulsive interactions between lone pairs of electrons on adjacent atoms. These interactions become important at the shorter internuclear distances encountered with the smaller, second-period elements of groups 15, 16, and 17. Stable compounds with N–N bonds are limited to chains of no more than three N atoms, such as the azide ion (N3−).
Nitrogen is the only pnicogen that normally forms multiple bonds with itself and other second-period elements, using π overlap of adjacent np orbitals. Thus the stable form of elemental nitrogen is N2, whose N≡N bond is so strong (DN≡N = 942 kJ/mol) compared with the N–N and N=N bonds (DN–N = 167 kJ/mol; DN=N = 418 kJ/mol) that all compounds containing N–N and N=N bonds are thermodynamically unstable with respect to the formation of N2. In fact, the formation of the N≡N bond is so thermodynamically favored that virtually all compounds containing N–N bonds are potentially explosive.
Again in contrast to carbon, nitrogen undergoes only two important chemical reactions at room temperature: it reacts with metallic lithium to form lithium nitride, and it is reduced to ammonia by certain microorganisms. At higher temperatures, however, N2 reacts with more electropositive elements, such as those in group 13, to give binary nitrides, which range from covalent to ionic in character. Like the corresponding compounds of carbon, binary compounds of nitrogen with oxygen, hydrogen, or other nonmetals are usually covalent molecular substances.
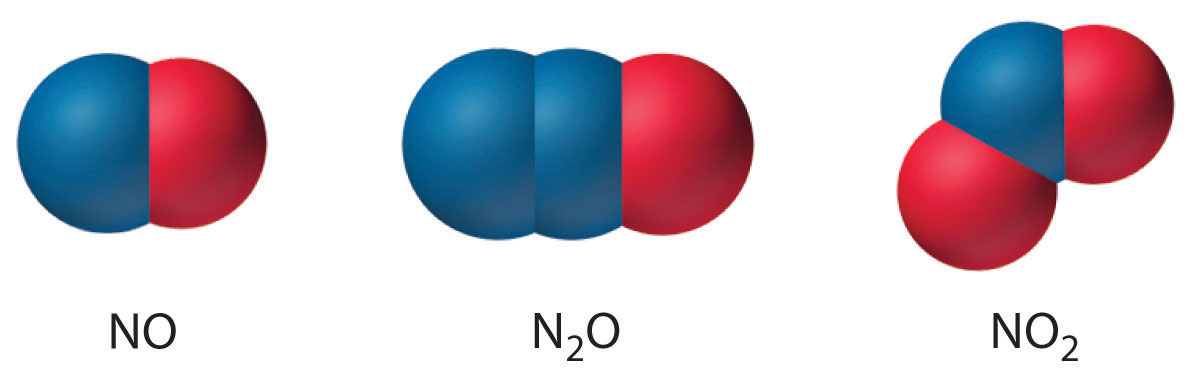
Few binary molecular compounds of nitrogen are formed by direct reaction of the elements. At elevated temperatures, N2 reacts with H2 to form ammonia, with O2 to form a mixture of NO and NO2, and with carbon to form cyanogen (N≡C–C≡N); elemental nitrogen does not react with the halogens or the other chalcogens. Nonetheless, all the binary nitrogen halides (NX3) are known. Except for NF3, all are toxic, thermodynamically unstable, and potentially explosive, and all are prepared by reacting the halogen with NH3 rather than N2. Both nitrogen monoxide (NO) and nitrogen dioxide (NO2) are thermodynamically unstable, with positive free energies of formation. Unlike NO, NO2 reacts readily with excess water, forming a 1:1 mixture of nitrous acid (HNO2) and nitric acid (HNO3):
Nitrogen also forms N2O (dinitrogen monoxide, or nitrous oxide), a linear molecule that is isoelectronic with CO2 and can be represented as −N=N+=O. Like the other two oxides of nitrogen, nitrous oxide is thermodynamically unstable. The structures of the three common oxides of nitrogen are as follows:
Few binary molecular compounds of nitrogen are formed by the direct reaction of the elements.
At elevated temperatures, nitrogen reacts with highly electropositive metals to form ionic nitrides, such as Li3N and Ca3N2. These compounds consist of ionic lattices formed by Mn+ and N3− ions. Just as boron forms interstitial borides and carbon forms interstitial carbides, with less electropositive metals nitrogen forms a range of interstitial nitrides, in which nitrogen occupies holes in a close-packed metallic structure. Like the interstitial carbides and borides, these substances are typically very hard, high-melting materials that have metallic luster and conductivity.
Nitrogen also reacts with semimetals at very high temperatures to produce covalent nitrides, such as Si3N4 and BN, which are solids with extended covalent network structures similar to those of graphite or diamond. Consequently, they are usually high melting and chemically inert materials.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















