


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-3-2017
Date: 23-3-2017
Date: 3-1-2017
|
Elemental boron is a semimetal that is remarkably unreactive; in contrast, the other group 13 elements all exhibit metallic properties and reactivity. We therefore consider the reactions and compounds of boron separately from those of other elements in the group. All group 13 elements have fewer valence electrons than valence orbitals, which generally results in delocalized, metallic bonding. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice with delocalized valence electrons. Instead, boron forms unique and intricate structures that contain multicenter bonds, in which a pair of electrons holds together three or more atoms.
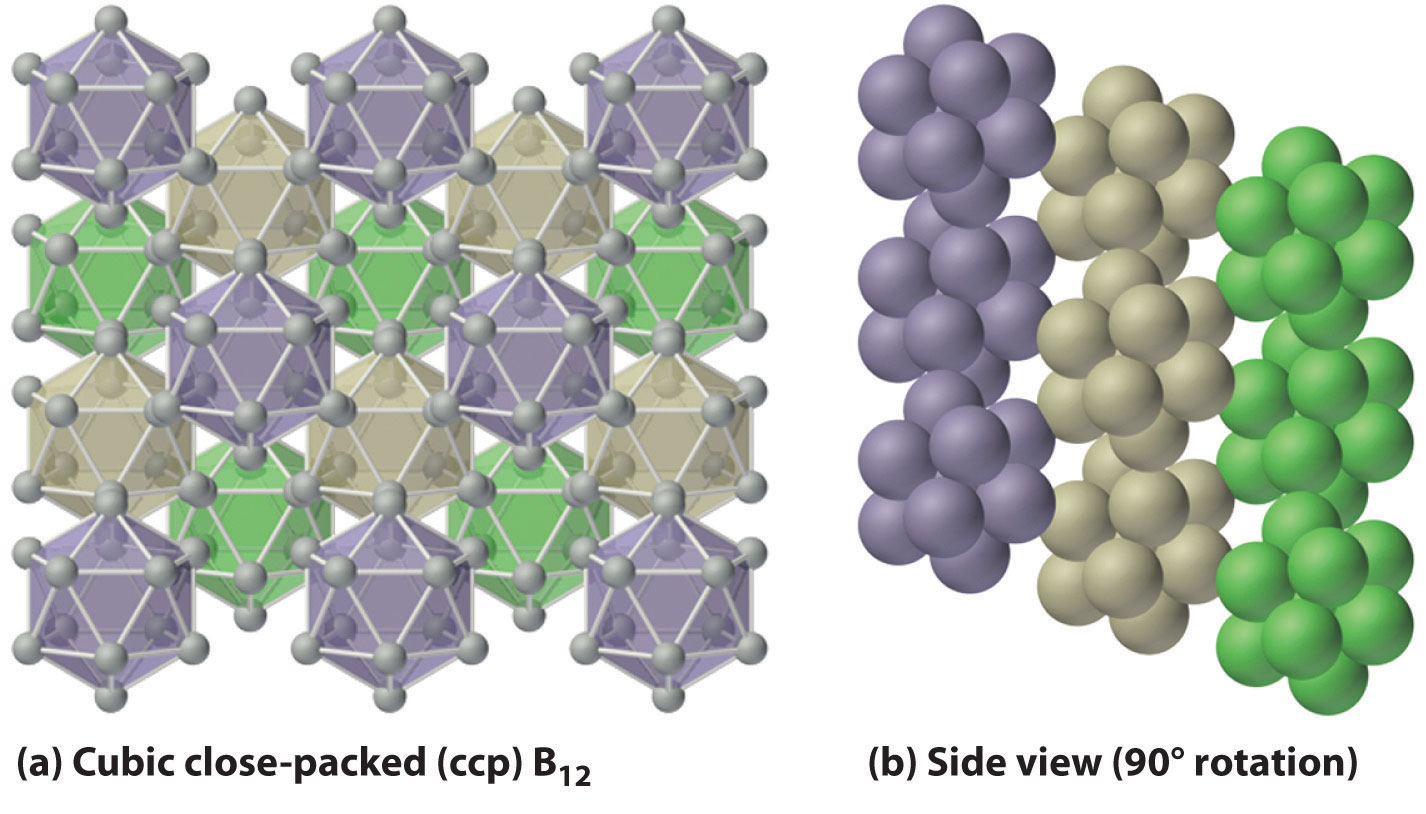
Figure 1 : Solid Boron Contains B12 Icosahedra. Unlike metallic solids, elemental boron consists of a regular array of B12 icosahedra rather than individual boron atoms. Note that each boron atom in the B12 icosahedron is connected to five other boron atoms within the B12 unit. (a) The allotrope of boron with the simplest structure is α-rhombohedral boron, which consists of B12 octahedra in an almost cubic close-packed lattice. (b) A side view of the structure shows that icosahedra do not pack as efficiently as spheres, making the density of solid boron less than expected.
Elemental boron forms multicenter bonds, whereas the other group 13 elements exhibit metallic bonding.
The basic building block of elemental boron is not the individual boron atom, as would be the case in a metal, but rather the B12 icosahedron. Because these icosahedra do not pack together very well, the structure of solid boron contains voids, resulting in its low density (Figure 1
). Elemental boron can be induced to react with many nonmetallic elements to give binary compounds that have a variety of applications. For example, plates of boron carbide (B4C) can stop a 30-caliber, armor-piercing bullet, yet they weigh 10%–30% less than conventional armor. Other important compounds of boron with nonmetals include boron nitride (BN), which is produced by heating boron with excess nitrogen (Equation 4); boron oxide (B2O3), which is formed when boron is heated with excess oxygen (Equation 5); and the boron trihalides (BX3), which are formed by heating boron with excess halogen (Equation 6 ).
Boron nitride is similar in many ways to elemental carbon. With eight electrons, the B–N unit is isoelectronic with the C–C unit, and B and N have the same average size and electronegativity as C. The most stable form of BN is similar to graphite, containing six-membered B3N3 rings arranged in layers. At high temperature and pressure, hexagonal BN converts to a cubic structure similar to diamond, which is one of the hardest substances known. Boron oxide (B2O3) contains layers of trigonal planar BO3 groups (analogous to BX3) in which the oxygen atoms bridge two boron atoms. It dissolves many metal and nonmetal oxides, including SiO2, to give a wide range of commercially important borosilicate glasses. A small amount of CoO gives the deep blue color characteristic of “cobalt blue” glass.

At high temperatures, boron also reacts with virtually all metals to give metal borides that contain regular three-dimensional networks, or clusters, of boron atoms. The structures of two metal borides—ScB12 and CaB6—are shown in Figure 2 . Because metal-rich borides such as ZrB2 and TiB2 are hard and corrosion resistant even at high temperatures, they are used in applications such as turbine blades and rocket nozzles.
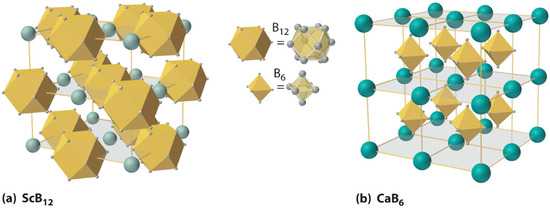
Figure 2 : The Structures of ScB12and CaB6, Two Boron-Rich Metal Borides. (a) The structure of ScB12 consists of B12 clusters and Sc atoms arranged in a faced-centered cubic lattice similar to that of NaCl, with B12units occupying the anion positions and scandium atoms the cation positions. The B12 units here are not icosahedra but cubooctahedra, with alternating square and triangular faces. (b) The structure of CaB6 consists of octahedral B6 clusters and calcium atoms arranged in a body-centered cubic lattice similar to that of CsCl, with B6 units occupying the anion positions and calcium atoms the cation positions.
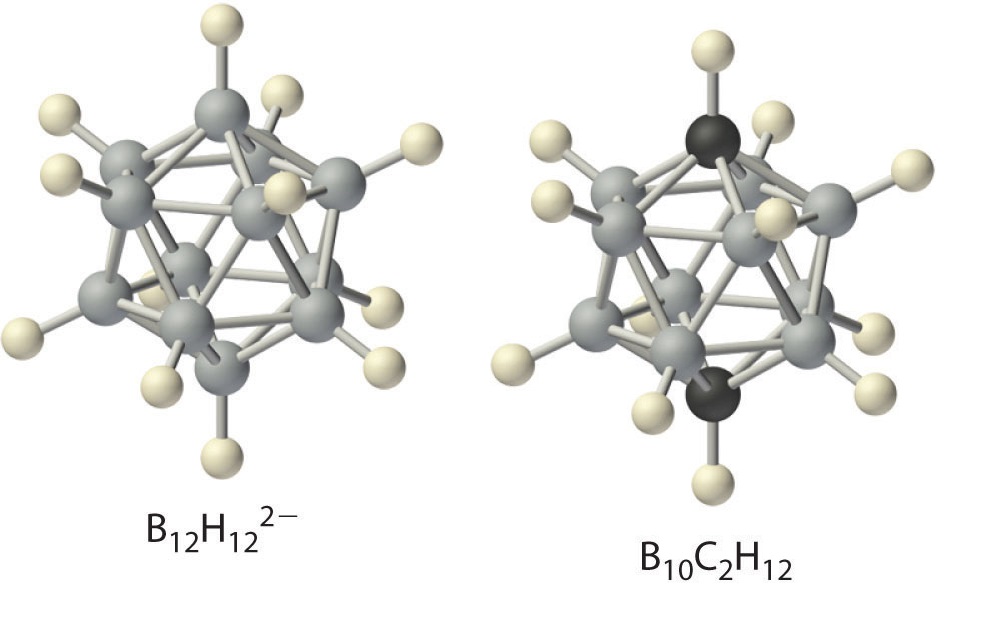
Boron hydrides were not discovered until the early 20th century, when the German chemist Alfred Stock undertook a systematic investigation of the binary compounds of boron and hydrogen, although binary hydrides of carbon, nitrogen, oxygen, and fluorine have been known since the 18th century. Between 1912 and 1936, Stock oversaw the preparation of a series of boron–hydrogen compounds with unprecedented structures that could not be explained with simple bonding theories. All these compounds contain multicenter bonds. The simplest example is diborane (B2H6), which contains two bridging hydrogen atoms (part (a) in Figure 3 . An extraordinary variety of polyhedral boron–hydrogen clusters is now known; one example is the B12H122− ion, which has a polyhedral structure similar to the icosahedral B12 unit of elemental boron, with a single hydrogen atom bonded to each boron atom.
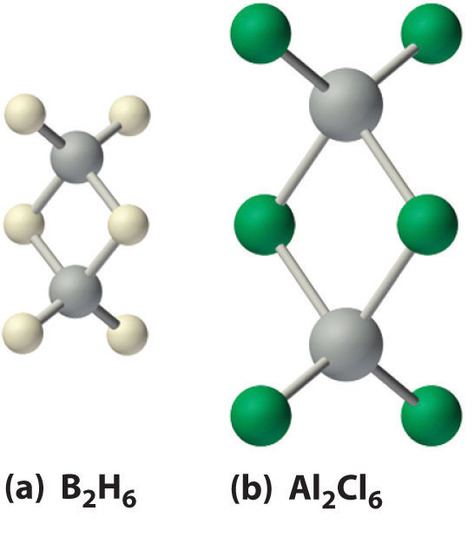
Figure 3 : The Structures of Diborane (B2H6) and Aluminum Chloride (Al2Cl6). (a) The hydrogen-bridged dimer B2H6 contains two three-center, two-electron bonds as described for the B2H7− ion in Figure 21.5. (b) In contrast, the bonding in the halogen-bridged dimer Al2Cl6 can be described in terms of electron-pair bonds, in which a chlorine atom bonded to one aluminum atom acts as a Lewis base by donating a lone pair of electrons to another aluminum atom, which acts as a Lewis acid.
A related class of polyhedral clusters, the carboranes, contain both CH and BH units; an example is shown here. Replacing the hydrogen atoms bonded to carbon with organic groups produces substances with novel properties, some of which are currently being investigated for their use as liquid crystals and in cancer chemotherapy.
The enthalpy of combustion of diborane (B2H6) is −2165 kJ/mol, one of the highest values known:
Consequently, the US military explored using boron hydrides as rocket fuels in the 1950s and 1960s. This effort was eventually abandoned because boron hydrides are unstable, costly, and toxic, and, most important, B2O3 proved to be highly abrasive to rocket nozzles. Reactions carried out during this investigation, however, showed that boron hydrides exhibit unusual reactivity.
Because boron and hydrogen have almost identical electronegativities, the reactions of boron hydrides are dictated by minor differences in the distribution of electron density in a given compound. In general, two distinct types of reaction are observed: electron-rich species such as the BH4− ion are reductants, whereas electron-deficient species such as B2H6 act as oxidants.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|