Theory of Accelerator Mass Spectroscopy
In conventional atomic mass spectrometry, samples are atomized and ionized, separated by their mass-to-charge ratio, then measured and/or counted by a detector. Rare isotopes such as 14C present a challenge to conventional MS due to their low natural abundance and high background levels. Researchers were challenged by isobaric interference (interference from equal mass isotopes of different elements exemplified by 14N in 14C analysis), isotopic interference (interference from equal mass to charge isotopes of different elements), and molecular interference (interference from equal mass to charge molecules, such as 12CH2-, 12CD, or 13CH- in 14C analysis). Most AMS systems employ an electrostatic tandem accelerator that has a direct improvement in background rejection, resulting in a 108 time increase in the sensitivity of isotope ratio measurements. As the natural abundance of 14C in modern carbon is 10-12 (isotopic ratio of 14C:12C), a sensitivity of 10-15 is a prerequisite for 14C analysis.
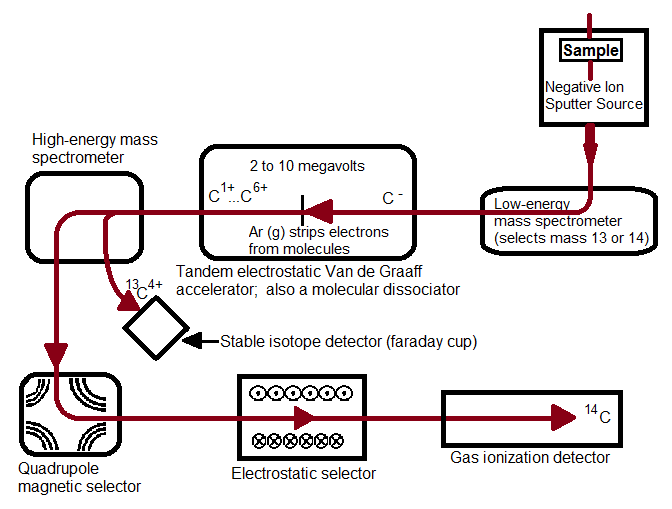
Figure 1. A schematic of the AMS system at Lawrence Livermore National Laboratory Center for Accelerator Mass Spectrometry.
Figure 1, above, starts with a negative ion sputter source, which commonly consists of a stream of Cesium ions (Cs+) with energies of 2-3 keV focused on the surface of a solid sample in order to transfer enough energy to the target material to produce free atoms and ions of the sample material. This process, called sputtering, separates neutral, as well as positive and negative ions from the sample surface. The sample is held at a negative potential, and negatively charged ions are accelerated away from the sample, resulting in a beam of negative ions (Figure 2, below). Cs+ is particularly useful in 14C studies because it does not form a negative ion from 14N, thereby eliminating isobar interference.[4] It is important to have a beam of negative ions entering the accelerator because the negative ions are attacted to the high -voltage terminal which results in their net acceleration.
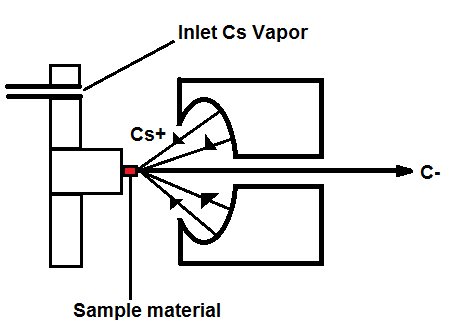
Figure 2. Cs sputter ion source.
The low energy (~5-10 keV) diverging beam that leaves the ion source is accelerated, focused and transported to the accelerator by the injector system.[2] CAMS LLNL employs a low-energy mass spectrometer that selects for the desired atomic mass[5] that separates ions by their mass to charge ratio (12C, 13C, and 14C ions pass through separately). Most AMS systems use sequential injection, a process that switches between stable and rare isotopes via the application of varying voltages to the electrically insulated vacuum chamber of the analyzer magnet. In sequential injection, typical injection repetition rates are 10 sec-1 to minimize variations in the electrical load.[2] This process allows the development of more versatile systems, allowing for analysis of a wide range of isotopes.[1] The alternative to sequential injection is simultaneous injection, a process adopted in accelerators dedicated to 14C analysis. A recombinator is used following sequential injection, which is a sequence of magnetic analyzers and quadrupole lenses that focus the stable and rare isotopes so they recombine and enter the accelerator together.
The traditional accelerator was first developed in the early 1930s for nuclear physics research. In 1939, UC Berkeley scientists Luis Alvarez and Robert Cornog were the first to use AMS in the detection of 3He in nature using the 88-inch Berkeley cylclotron.[5] Now, over 70 years later, cyclotrons have been replaced by an accelerator type with greater energy stability: the tandem electrostatic accelerator. An electrostatic accelerator works by accellerating particles though a magnetic field generated by high voltages using a mechanic transport system that continuously transports charges from ground to the insulated high-voltage terminal. All tandem accelerators with a maximum terminal voltage above 5 MV use such a mechanical system.[2] The negative ions that enter the accelerator are attracted to the high-voltage terminal, which is what accellerates theCAMS LLNL employs a tandem Van de Graaff accelerator, in which a second acceleration of millions of volts is applied. In all tandem accelerators, atoms are stripped at the high-voltage terminal using either a thin Carbon foil or Argon gas. Stripping is the process in which two or more electrons are removed. The Van de Graaff accelerator removes at least four electrons. It is preferrable to remove at least three electrons because by this process that molecular isobars of 14C (such as 12CH2-, 12CD, or 13CH-) are destroyed due to the high instability of their positively charged forms, and atomic C+ ions such as 12C+, 13C+, and 14C+ are separated due to their different mass to charge ratios.[4] The negative ions are changed to positively charged ions and are thus accelerated back to the ground potential in the high-energy part of the accelerator. Transmission through a foil changes with time due to radiation damage and foil thickening, thus gas strippers are used in all modern analyzers due to their increased transmission stability.
Magnetic lenses focus the high energy particles leaving the accelerator into a magnetic dipole, (the high energy analyzing magnet). Stable isotopes can be collected at off-axis beam stops where secondary focusing lenses and additional analyzing equipment remove unwanted ions and molecular fragments to eliminate background. At CAMS LLNL, a magnetic quadrupole lens focuses the desired isotope and charge state to a high-energy mass spectrometer which passes 12C+ and 13C+ into Faraday cups and further focuses and stabilizes 14C in a quadrupole/electrostatic cylindrical analyzer that leads to a gas ionization detector.[5] The magnetic quadrupole and electrostatic selectors coupled together ensure high selectivity and sensitivity, respectively. Other detectors commonly found in AMS systems include surface barrier, time-of-flight, gas filled magnets, and x-ray detectors.


