
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 29-8-2019
Date: 7-7-2016
Date: 8-10-2018
|
With an alkyl amine the lone pair electron is localized on the nitrogen. However, the lone pair electron on an amide are delocalized between the nitrogen and the oxygen through resonance. This makes amides much less basic compared to alkylamines.
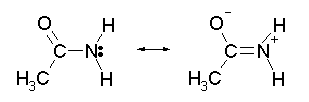
In fact,when and amide is reacted with an acid, the protonation occurs at the carbonyl oxygen and not the nitrogen. This is because the cation resulting from oxygen protonation is resonance stabilized. The cation resulting for the protonation of nitrogen is not resonance stabilized.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تستعد لإطلاق الحفل المركزي لتخرج طلبة الجامعات العراقية
|
|
|