

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Stereoelectronic Control of Enolization
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
8-10-2018
1498
Stereoelectronic Control of Enolization
Many carbon acids have enhanced acidity because of a neighboring functional group. The acidity of alpha hydrogens in aldehydes, ketones and esters is well documented, and is the source of many important synthetic procedures. The following equation illustrates the general enolate anion transformation, with the acidic alpha-hydrogen colored red. The resulting ambident anion is stabilized by charge delocalization, and may react with electrophiles at both carbon and oxygen.
Stereoelectronic factors govern the enolization reaction, as illustrated by clicking on the diagram below. The bond from the alpha carbon to the acidic alpha-hydrogen must be oriented 90º to the plane of the carbonyl group, or parallel to the pi-electron system (colored magenta here). The ideal overlap occurs with a 0º dihedral angle between this bond and the pi-orbital, as shown.
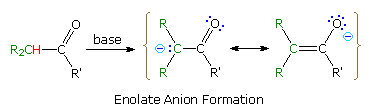
By clicking on the diagram a second time, the importance of this stereoelectronic requirement will be demonstrated. An increase in the acidity of carbon acids activated by two carbonyl groups is well known, and is illustrated by the two beta-dicarbonyl compounds on the left side of the diagram. In such cases the acidic C-H unit may be oriented perpendicular to both carbonyl groups, and the resulting planar anion is stabilized by additional charge delocalization (over both oxygens and the central carbon). In the case of the bicyclic diketone on the right, the C-H bond nearly eclipses the two carbonyl C-O bonds, resulting in a dihedral angle with the pi-electron systems of roughly 90º. Consequently, the acidity of this hydrogen is similar to that of the hydrogens of an alkane or cycloalkane. It should also be apparent that if an enolate anion were to be formed to the bridgehead carbon, the double bond would be prohibited by Bredt's rule.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















