

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Alcohols
المؤلف:
..................
المصدر:
LibreTexts Project
الجزء والصفحة:
.................
2-9-2019
1310
Alcohols
Molecules of alcohols contain one or more hydroxyl groups (OH groups) substituted for hydrogen atoms along the carbon chain. The structure of the simplest alcohol, methanol (methyl alcohol), can be derived from that of methane by putting an OH in place of one of the H’s:
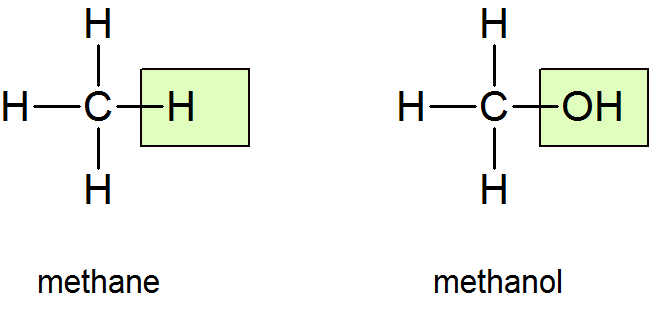
The name, too, is derived from the name methane by replacing the final e with ol (for alcohol). The general formula for an alcohol may be written as R—OH, where R represents the hydrocarbon (alkane) portion of the molecule and is called an alkyl group. In methanol, R is the methyl group CH3.
Methanol is also called wood alcohol because it can be obtained by heating wood in the absence of air, a process called destructive distillation. Methanol vapor given off when the wood is heated can be condensed to a liquid by cooling below its boiling point of 65°C. The effect of polarity and especially hydrogen bonding due to the OH group is evident when this is compared with the temperature of –85°C at which ethane, C2H6, boils. Both molecules contain 18 electrons and are nearly the same size, and so London forces should be about the same, but the OH group in one methanol molecule can form strong hydrogen bonds with an OH in another molecule. Methanol is an important industrial chemical—nearly 3 × 1010 kg was produced worldwide in 2003. Some was made by destructive distillation, but most was synthesized from hydrogen and carbon monoxide:
This reaction is carried out at pressures several hundred times normal atmospheric pressure, using metal oxides as catalysts. Methanol is mainly used to make other compounds from which plastics are manufactured, but some is consumed as fuel in jet engines and racing cars. Methanol is also a component of nonpermanent antifreeze and automobile windshield-washer solvent.
The second member of the alcohol family is ethanol (ethyl alcohol)― the substance we commonly call alcohol. Ethanol is also known as grain alcohol because it is obtained when grain or sugar ferments. Fermentation refers to a chemical reaction which is speeded up by enzymes and occurs in the absence of air. (Enzymes, catalysts which occur naturally in yeasts and other living organisms, are discussed in more detail elsewhere.)
Ethanol can also be synthesized by adding H2O to ethene, obtained during petroleum refining:
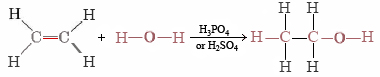
This is a typical example of an addition reaction. The H and OH from H2O are added to the ethene molecule and held there by electrons made available when one-half of the double bond breaks.
Ethanol is used as a solvent, in some special fuels, in antifreeze, and to manufacture a number of other chemicals. You are probably most familiar with it as a component of alcoholic beverages. Ethanol makes up 3 to 6 percent of beer, 12 to 15 percent of most wines, and 49 to 59 percent of distilled liquor. (The “proof” of an alcoholic beverage is just twice the percentage of ethanol.) Alcohol’s intoxicating effects are well known, and it is a mild depressant. Prolonged overuse can lead to liver damage. Methanol also produces intoxication but is much more poisonous than ethanol—it can cause blindness and death. Denatured alcohol is ethanol to which methanol or some other poison has been added, making it unfit for human consumption. Most of the ethanol not used in alcoholic beverages is denatured because in that form its sale is taxed at a much lower rate.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















