

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Sterically Hindered Substrates Will Reduce the SN2 Reaction Rate
المؤلف:
..................
المصدر:
LibreTexts Project
الجزء والصفحة:
.................
31-7-2019
1602
Sterically Hindered Substrates Will Reduce the SN2 Reaction Rate
Now that we have discussed the effects that the leaving group, nucleophile, and solvent have on biomolecular nucleophilic substitution (SN2) reactions, it's time to turn our attention to how the substrate affects the reaction. Although the substrate, in the case of nucleophilic substitution of haloalkanes, is considered to be the entire molecule circled below, we will be paying particular attention to the alkyl portion of the substrate. In other words, we are most interested in the electrophilic center that bears the leaving group.
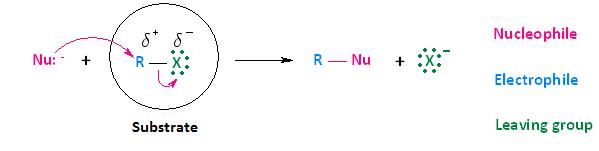
In the section Kinetics of Nucleophilic Substitution Reactions, we learned that the SN2 transition state is very crowded. Recall that there are a total of five groups around the electrophilic center, the nucleophile, the leaving group, and three substituents.
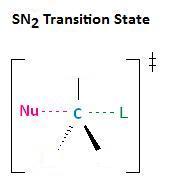
If each of the three substituents in this transition state were small hydrogen atoms, as illustrated in the first example below, there would be little steric repulsion between the incoming nucleophile and the electrophilic center, thereby increasing the ease at which the nucleophilic substitution reaction can occur. Remember, for the SN2 reaction to occur, the nucleophile must be able to attack the electrophilic center, resulting in the expulsion of the leaving group. If one of the hydrogens, however, were replaced with an R group, such as a methyl or ethyl group, there would be an increase in steric repulsion with the incoming nucleophile. If two of the hydrogens were replaced by R groups, there would be an even greater increase in steric repulsion with the incoming nucleophile.

How does steric hindrance affect the rate at which an SN2 reaction will occur? As each hydrogen is replaced by an R group, the rate of reaction is significantly diminished. This is because the addition of one or two R groups shields the backside of the electrophilic carbon, impeding nucleophilic attack.
The diagram below illustrates this concept, showing that electrophilic carbons attached to three hydrogen atoms results in faster nucleophilic substitution reactions, in comparison to primary and secondary haloalkanes, which result in nucleophilic substitution reactions that occur at slower or much slower rates, respectively. Notice that a tertiary haloalkane, that which has three R groups attached, does not undergo nucleophilic substitution reactions at all. The addition of a third R group to this molecule creates a carbon that is entirely blocked.

 الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















