


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-7-2019
Date: 22-9-2019
Date: 15-1-2020
|
With respect to electronegativity, halogens are more electronegative than carbons. This results in a carbon-halogen bond that is polarized. As shown in the image below, carbon atom has a partial positive charge, while the halogen has a partial negative charge.
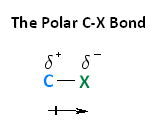
The following image shows the relationship between the halogens and electronegativity. Notice, as we move up the periodic table from iodine to fluorine, electronegativity increases.
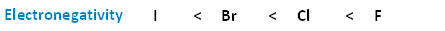
The following image shows the relationships between bond length, bond strength, and molecular size. As we progress down the periodic table from fluorine to iodine, molecular size increases. As a result, we also see an increase in bond length. Conversely, as molecular size increases and we get longer bonds, the strength of those bonds decreases.




|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|