
Unique Chemistry of the Lightest Elements
 المؤلف:
..................
المؤلف:
..................
 المصدر:
LibreTexts Project
المصدر:
LibreTexts Project
 الجزء والصفحة:
.................
الجزء والصفحة:
.................
 15-6-2019
15-6-2019
 1607
1607
Unique Chemistry of the Lightest Elements
The chemistry of the second-period element of each group (n = 2: Li, Be, B, C, N, O, and F) differs in many important respects from that of the heavier members, or congeners, of the group. Consequently, the elements of the third period (n = 3: Na, Mg, Al, Si, P, S, and Cl) are generally more representative of the group to which they belong. The anomalous chemistry of second-period elements results from three important characteristics: small radii, energetically unavailable d orbitals, and a tendency to form pi (π) bonds with other atoms.
In contrast to the chemistry of the second-period elements, the chemistry of the third-period elements is more representative of the chemistry of the respective group.
Due to their small radii, second-period elements have electron affinities that are less negative than would be predicted from general periodic trends. When an electron is added to such a small atom, increased electron–electron repulsions tend to destabilize the anion. Moreover, the small sizes of these elements prevent them from forming compounds in which they have more than four nearest neighbors. Thus BF3 forms only the four-coordinate, tetrahedral BF4− ion, whereas under the same conditions AlF3 forms the six-coordinate, octahedral AlF63− ion. Because of the smaller atomic size, simple binary ionic compounds of second-period elements also have more covalent character than the corresponding compounds formed from their heavier congeners. The very small cations derived from second-period elements have a high charge-to-radius ratio and can therefore polarize the filled valence shell of an anion. As such, the bonding in such compounds has a significant covalent component, giving the compounds properties that can differ significantly from those expected for simple ionic compounds. As an example, LiCl, which is partially covalent in character, is much more soluble than NaCl in solvents with a relatively low dielectric constant, such as ethanol (ε = 25.3 versus 80.1 for H2O).
Because d orbitals are never occupied for principal quantum numbers less than 3, the valence electrons of second-period elements occupy 2s and 2p orbitals only. The energy of the 3d orbitals far exceeds the energy of the 2s and 2p orbitals, so using them in bonding is energetically prohibitive. Consequently, electron configurations with more than four electron pairs around a central, second-period element are simply not observed. You may recall that the role of d orbitals in bonding in main group compounds with coordination numbers of 5 or higher remains somewhat controversial. In fact, theoretical descriptions of the bonding in molecules such as SF6 have been published without mentioning the participation of d orbitals on sulfur. Arguments based on d-orbital availability and on the small size of the central atom, however, predict that coordination numbers greater than 4 are unusual for the elements of the second period, which is in agreement with experimental results.
One of the most dramatic differences between the lightest main group elements and their heavier congeners is the tendency of the second-period elements to form species that contain multiple bonds. For example, N is just above P in group 15: N2 contains an N≡N bond, but each phosphorus atom in tetrahedral P4 forms three P–P bonds. This difference in behavior reflects the fact that within the same group of the periodic table, the relative energies of the π bond and the sigma (σ) bond differ. A C=C bond, for example, is approximately 80% stronger than a C–C bond. In contrast, an Si=Si bond, with less p-orbital overlap between the valence orbitals of the bonded atoms because of the larger atomic size, is only about 40% stronger than an Si–Si bond. Consequently, compounds that contain both multiple and single C to C bonds are common for carbon, but compounds that contain only sigma Si–Si bonds are more energetically favorable for silicon and the other third-period elements.
Another important trend to note in main group chemistry is the chemical similarity between the lightest element of one group and the element immediately below and to the right of it in the next group, a phenomenon known as the diagonal effect (Figure 1.1) There are, for example, significant similarities between the chemistry of Li and Mg, Be and Al, and B and Si. Both BeCl2 and AlCl3 have substantial covalent character, so they are somewhat soluble in nonpolar organic solvents. In contrast, although Mg and Be are in the same group, MgCl2 behaves like a typical ionic halide due to the lower electronegativity and larger size of magnesium.
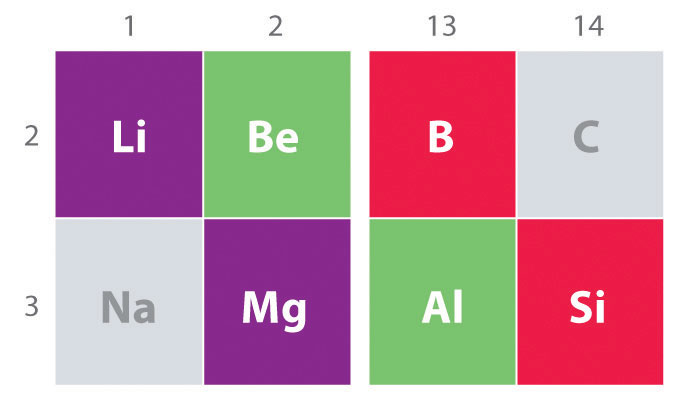
Figure 1.1
: The Diagonal Effect. The properties of the lightest element in a group are often more similar to those of the element below and to the right in the periodic table. For instance, the chemistry of lithium is more similar to that of magnesium in group 2 than it is to the chemistry of sodium, the next member in group 1.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


