
The structure of carbon dioxide
 المؤلف:
........
المؤلف:
........
 المصدر:
LibreTexts Project
المصدر:
LibreTexts Project
 الجزء والصفحة:
............
الجزء والصفحة:
............
 1-4-2019
1-4-2019
 2032
2032
The structure of carbon dioxide
The fact that carbon dioxide is a gas indicates that it consists of small, simple molecules. Carbon can form these molecules because it can form double bonds with oxygen.
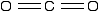
None of the other elements in Group 4 form double bonds with oxygen, so their oxides adopt completely different structures. When carbon forms bonds with oxygen, it promotes one of its 2s electrons into the empty 2p level. This produces 4 unpaired electrons.
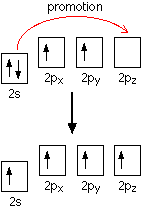
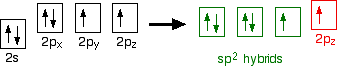
These electrons are rearranged by hybridizing the 2s electron and one of the 2p electrons to make two sp1 hybrid orbitals of equal energy. The other 2p electrons are unaffected during this process.
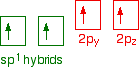
The figure below illustrates this:
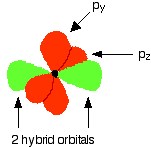
Notice that the two green lobes are two different hybrid orbitals, arranged as far from each other as possible (although the two hybrid orbitals have an arrangement similar to a p orbital, it is important not to confuse the two).
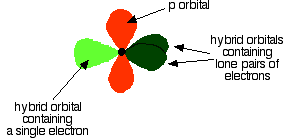
Oxygen's electronic structure is 1s22s22px22py12pz1, and its orbitals must also hybridize. In this case, sp2 hybrids are formed from the s orbital and two of the p orbitals, rearranging to form 3 orbitals of equal energy, leaving a temporarily unaffected p orbital.
As shown below, two of the sp2 hybrid orbitals contain lone pairs of electrons.
In the figure below, the carbon and oxygen atoms are arranged in pre-bonding position:
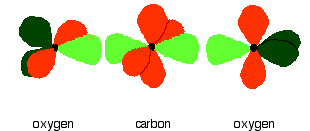
The green hybrid orbitals overlap end-to-end, forming covalent bonds. These are called sigma bonds, and are shown as orange in the next diagram. The sigma bonding brings the p orbitals close enough to overlap.
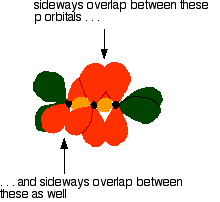
This overlap between the two sets of p orbitals produces two pi
bonds, similar to the pi bond found in ethene. These pi
bonds are twisted at 90° to each other in the final molecule.
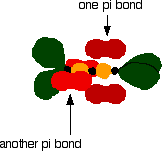
To form a carbon-oxygen double bond, it is necessary for the lobes of the p orbitals on the carbon and the oxygen to overlap correctly.
 الاكثر قراءة في مقالات متنوعة في علم الكيمياء
الاكثر قراءة في مقالات متنوعة في علم الكيمياء
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


