


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 8-2-2019
Date: 8-2-2019
Date: 8-2-2019
|
"Normal" rainfall is slightly acidic because of the presence of dissolved carbonic acid. Carbonic acid is the same as that found in soda pop.
The pH of "normal" rain has traditionally been given a value of 5.6. However scientists now believe that the pH of rain may vary from 5.6 to a low of 4.5 with the average value of 5.0. Acid rain or acid snow is a direct result of the method that the atmosphere cleans itself. The tiny droplets of water that make up clouds, continuously capture suspended solid particles and gases in the atmosphere. The gases of sulfur oxides and nitrogen oxides are chemically converted into sulfuric and nitric acids. The non-metal oxide gases react with water to produce acids (ammonia produces a base).
When enough of the tiny cloud droplets clump together to form a larger water drop it may fall to the earth as "wet" acid precipitation including rain, snow, ice, sleet, or fog. See Acids and Bases for more details.
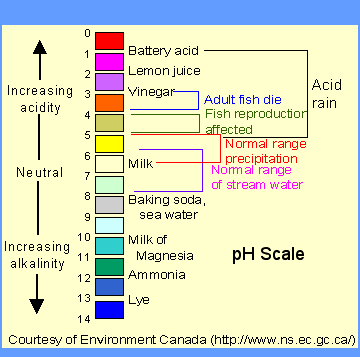



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|