


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 1-4-2019
Date: 11-1-2019
Date: 25-1-2019
|
Silicon is most commonly found in silicate compounds. Silica is the one stable oxide of silicon, and has the empirical formula SiO2. Silica is not a silicon atom with two double bonds to two oxygen atoms. Silica is composed of one silicon atom with four single bonds to four oxygen molecules (Figure 1).
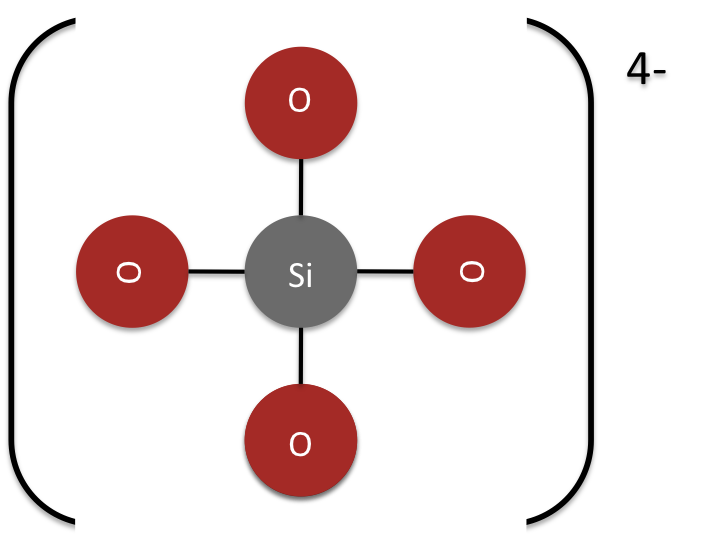
Figure 1: The net charge of silica is minus 4
Silica, i.e. silicon dioxide, takes on this molecular form, instead of carbon dioxide's characteristic shape, because silicon's 3p orbitals make it more energetically favorable to create four single bonds with each oxygen rather than make two double bonds with each oxygen atom. This leads to silicates linking together in -Si-O-Si-O- networks called silicates. The empirical form of silica is SiO2 because, with respect to the net average of the silicate, each silicon atom has two oxygen atoms.
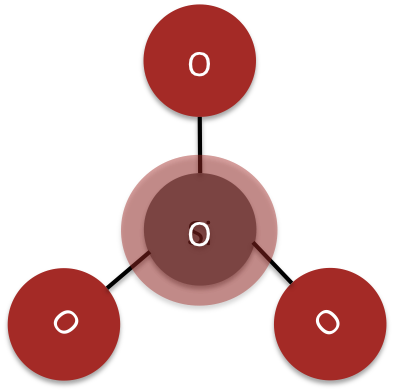
Figure 2: This is a representation of the tetrahedral silica complex
The tetrahedral SiO44- complex (see Figure 2), the core unit of silicates, can bind together in a variety of ways, creating a wide array of minerals. Silicon is an integral component in minerals, just as Carbon is an essential component of organic compounds.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|