

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
SN2 Reactions
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
8-10-2018
4913
SN2 Reactions
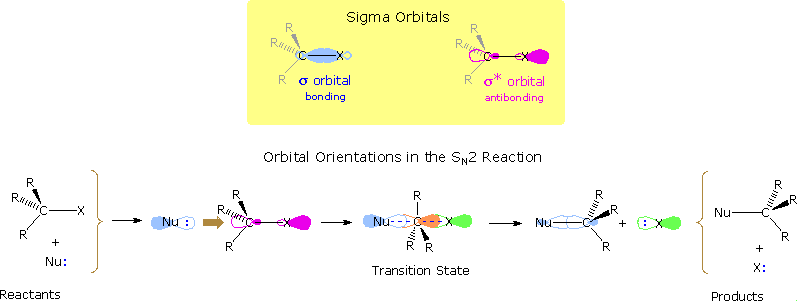
Nucleophilic substitution reactions of alkyl halides take place by a continuum of mechanisms, defined by SN2 behavior at one extreme and SN1 behavior at the other. The essential characteristics of the SN2 process include inversion of configuration at the reaction site, sensitivity to steric hindrance in the alkyl group, and second order kinetic behavior. The orbital interactions that take place in a successful SN2 reaction are shown in the following diagram. The bonding and antibonding sigma molecular orbitals composing the C-X bond are drawn in the yellow box at the top of the diagram. As a nucleophile approaches the rear side of the carbon, the orbital containing the non-bonded electron pair (light blue) begins to overlap with the empty antibonding C-X sigma orbital (pink colored), shown on the left of the bottom line. As electrons occupy this antibonding orbital, the C-X bond is weakened. In the transition state partial bonds exists between the carbon and both the nucleophile and the halogen (the carbon is essentially sp2 hybridized with the orange colored p-orbital providing the partial bonding). Finally, a full C-Nu sigma bond develops and the halogen leaves as an anion.
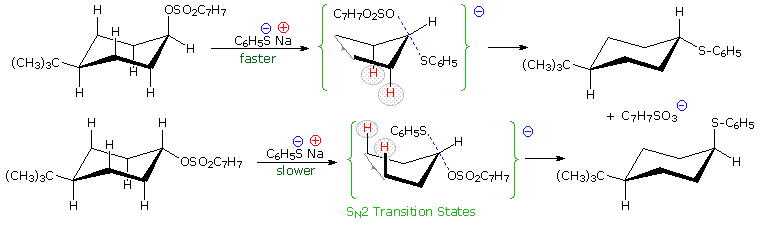
Other substitution reactions also proceed by the SN2 pathway, as illustrated by the stereoisomeric cyclohexyl tosylates in the following diagram. The bulky tert-butyl group on C-4 serves to lock the chair conformation in the configuration shown, so one isomer has an equatorial tosylate function, and the other an axial tosylate. On reaction with sodium thiophenolate, both isomers undergo bimolecular substitution with inversion, the axial isomer reacting about thirty times faster than its equatorial epimer. Steric hindrance to rear side nucleophile approach by the red colored hydrogen atoms is present for each isomer, but the relief of steric crowding of the axial tosylate leaving group helps to facilitate substitution of the that isomer.

SN2 reactions may be intermolecular, as in these examples, or intramolecular. By clicking on the above equations, an example of two sequential substitutions, the first intermolecular and the second intramolecular, will be displayed. Note that the intramolecular substitution shown here proceeds via the same orbital alignment described above. The reacting moieties in intramolecular reactions are incorporated in the same molecule; consequently, such reactions exhibit first order kinetic behavior instead of the usual second order kinetics. Because enolate alkylation reactions are irreversible, the strained four-membered ring product is stable under the reaction conditions.
In general, intramolecular forms of bimolecular reactions are faster than their intermolecular counterparts, since the reactive sites are held close together (their relative concentrations are as high as possible). The rapid lactonization of gamma and delta-hydroxy acids compared with corresponding intermolecular esterifications is another example of this principle, which is associated with a favorable entropy change. Note, however, that ring size has a profound influence on the intra- vs. intermolecular dichotomy.
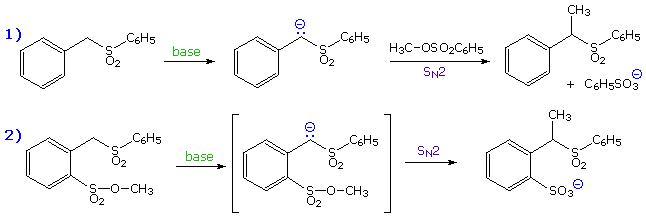
Maximum overlap of the electron orbital of the nucleophile with the antibonding sigma orbital of the carbon substituent requires an approach from the rear side of the carbon, ideally 180º from the leaving group. The importance of this orientation, which is shown in previous diagrams, was made clear by an experiment conducted by A. Eschenmoser over 25 years ago. The results of Eschenmoser's experiment are presented in the following illustration. Initially, two equations are written. The first describes the intermolecular methylation of a sulfone anion by methyl tosylate, a typical SN2 reaction. The second is a very similar reaction which many would consider to be intramolecular, since a methyl sulfonate moiety is positioned ortho to the sulfone function.
By clicking on these equations, evidence against the intramolecular mechanism will be displayed. Although intramolecular reactions are often favored, this case requires a significant departure from the 180º orbital alignment required by an authentic SN2 reaction. Since this reaction was found to be second order and is not faster than the simple intermolecular analog (reaction 1 on the preceding slide), it cannot be intramolecular.

 الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















