

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
[2+2]-Cycloaddition
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
2-9-2018
2842
[2+2]-Cycloaddition
In the earlier discussion of stilbene photoisomerization a [2+2]-cyclodimerization was noted. The construction of cyclobutane rings from alkene components is rare in ground state chemistry, so a general photochemical synthesis of this kind would provide a valuable addition to our assortment of useful carbon-carbon bond forming reactions. Double bonds activated by conjugation with a carbonyl group have proven especially effective in this respect, both in forming [2+2] dimers and adding to isolated alkene functions. A particularly important example of photodimerization involves the damage to DNA caused by uv light. Thymine (or uracil in the case of RNA) is one of four heterocyclic base components of nucleic acids. On exposure to ultraviolet light, thymine may undergo [2+2]-dimerization with a suitably oriented nearby thymine, as shown in the diagram below. When this happens toxic products are generated that are directly responsible for cell death via mutagenic action, suppression of DNA transformation and/or activation of carcinogenic pathways. A natural repair mechanism employing a photolyase enzyme system reverses the dimerization.
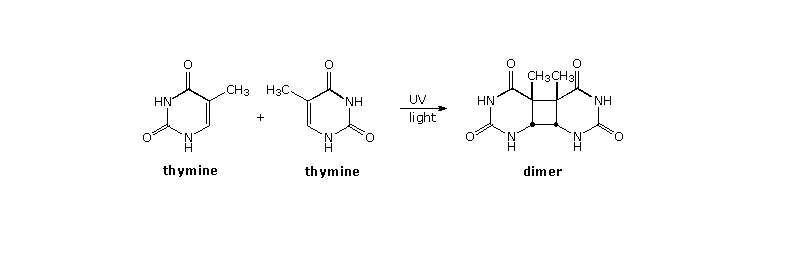
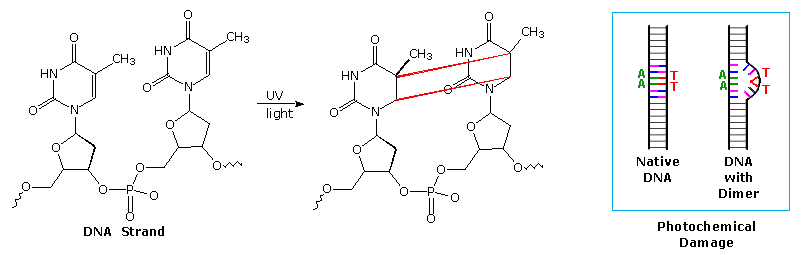
Four possible dimers of thymine (and uracil) may be formed. The cis-syn isomer shown above is the chief dimer formed by irradiation of DNA, or from frozen aqueous solutions of thymine or uracil. Other isomers are formed in varying amounts from room temperature solutions of these bases. By clicking on the diagram a drawing showing the pertubation of DNA by this dimerization will be displayed.
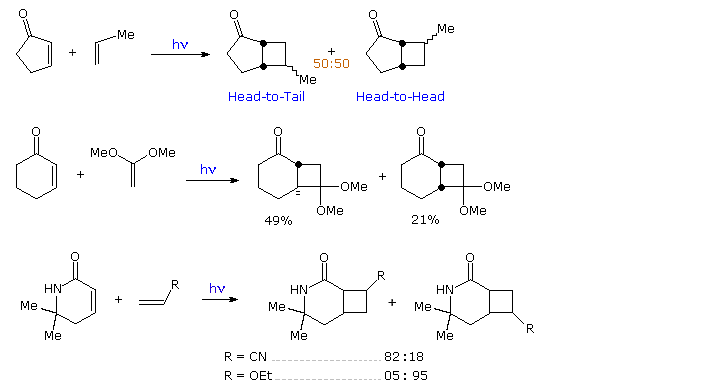
In reactions with unsymmetrically substituted alkenes, cyclic enones yield fused cyclobutane rings with variable regio and stereoselectivity. As shown in the following diagram, the addition of 2-cyclopentenone to propene gives a mixture of regioisomers, both having a cis-fused bicyclo [3,2,0] heptane unit. In contrast, addition of 2-cyclohexenone to 1,1-dimethoxyethene proceeds with good regioselectivity and modest stereoselectivity favoring a trans-ring fusion. The last example demonstrates that unsaturated carbonyl functions other than ketones may lead to cycloaddition products, and that functional substituents on the alkene moiety may influence the regioselectivity.
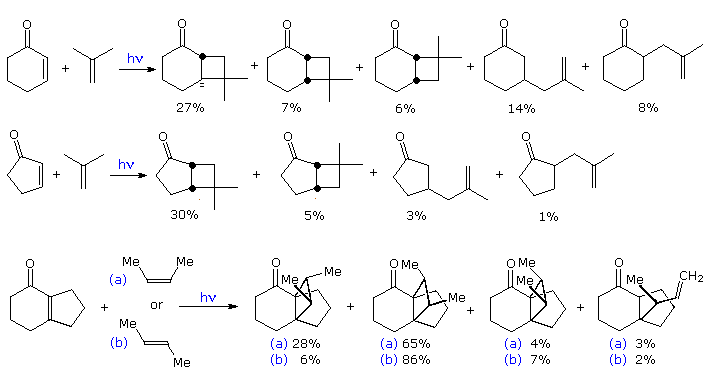
By clicking on the diagram, three more examples will be displayed, The first two confirm the modest [2+2] regioselectivity noted in the first display and show additional monocyclic products. The third reaction discloses there is very low retention of configuration in the cycloaddition of alkene stereoisomers. This stands in marked contrast to the high selectivity that characterizes [4+2] cycloadditions known as the Diels-Alder reaction. A similar lack of stereoselectivity is found in intramolecular [2+2] cycloadditions, as may be seen by clicking the diagram a second time. Three additional examples of the intramolecular variant (I. II & III) are also presented in this new display. The importance of this method for the assembly of complex molecules is shown by the short efficient synthesis of the sesquiterpene isocomene accomplished by M. Pirrung.
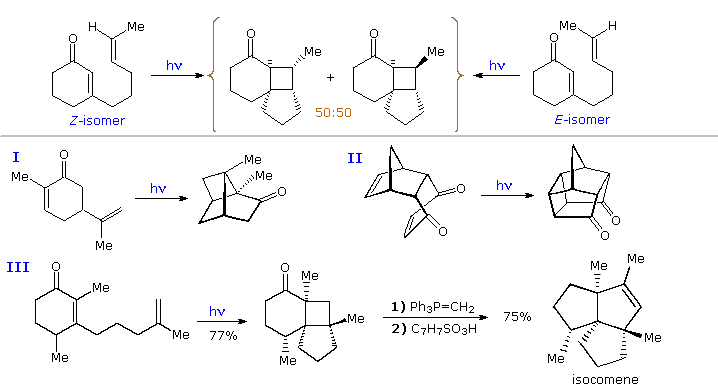
The poor selectivities and modest quantum yields observed for these cycloaddition reactions have engendered several mechanistic interpretations. Identification of the enone excited state as a π → π* triplet comes from selective quenching studies as well as transient absorption spectroscopy. Bauslaugh's proposal that this triplet adds to the alkene to form a 1,4-biradical proposal is still the simplest and most satisfying explanation of the facts. As shown in the following diagram, the initial bonding may take place at either the α or β-carbon of the enone. The resulting biradicals would also be triplets, and on intersystem crossing to a singlet could yield ground state products by one of three paths.
| (i) Cyclization to a fused cyclobutane product. |
| (ii) Disproportionation by a 1,5-hydrogen transfer, yielding an α or β alkenyl-substituted ketone. |
| (iii) Fragmentation back to the starting compounds |
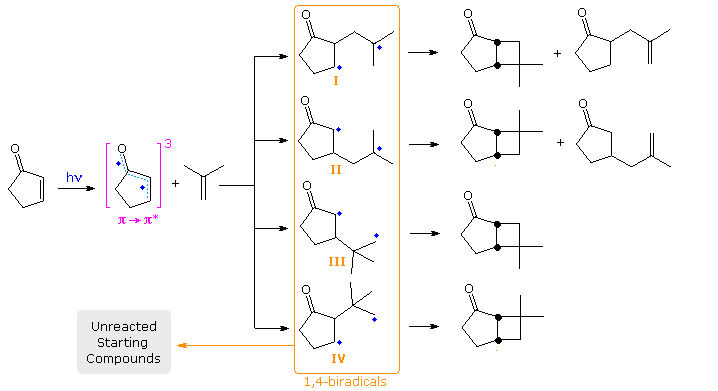
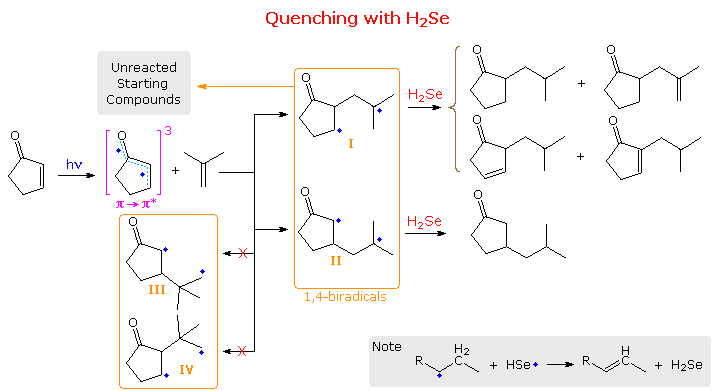
The latter event accounts for low quantum yields, and is presumed to be fast because isomerization of recovered alkene is small when cis and trans-2-butene are used as the alkene reactant. For the addition of cyclopentenone to isobutene, four possible biradicals may be drawn, the estimated stability order being II > I > III > IV. Finally, although the biradical species are very short lived (ca. 60 ns), it has been possible to trap them by use of the foul-smelling, highly-toxic, hydrogen donor hydrogen selenide. The results of such a trapping study will be displayed below by clicking on the diagram.
Several important conclusions were reached in this case. First, no initial addition to the more substituted carbon of the alkene took place, since tert-butyl substituted cyclopentanones would have been produced (via III & IV) and none were found. Second, although initial bonding at the β-carbon (yielding II) was nearly twice as fast as α-bonding, over 96% of II fragmented to starting materials, compared with 79% of I. Consequently most of the trapped products came from I. These products were formed either by hydrogen atom delivery to both radical sites (giving saturated compounds) or hydrogen delivery followed by hydrogen abstraction by HSe•. (giving unsaturated compounds), as noted in the gray-shaded box bottom-right..
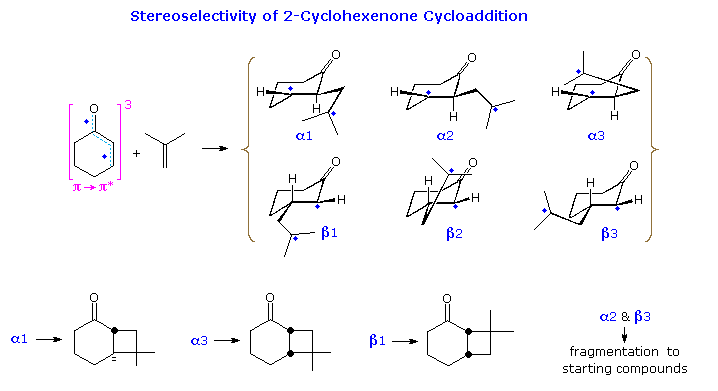
The formation of trans-fused bicyclo [4,2,0] octane products from 2-cyclohexenone has been explained by considering the conformations of the 1,4-biradical intermediates. This will be illustrated above .
Here, three rotamers (1, 2 & 3) for both the α and β chains are drawn. When the radical sites are far apart (α2 and β3), fragmentation of the biradical is likely. The trans-ring fusion will be formed from conformation α1, and cis from α3. The latter bonding will be subject to some axial hindrance. For the β-manifold, β1 should yield a cis-fused product and β2 the trans-isomer. However the latter bonding would again be hindered.
 الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















